Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Salud Pública de México
versão impressa ISSN 0036-3634
Salud pública Méx vol.57 no.2 Cuernavaca Mar./Abr. 2015
Artículo breve
Primary drug resistance in a region with high burden of tuberculosis. A critical problem
Farmacorresistencia en casos nuevos en una región con alta carga de tuberculosis. Un problema crítico
Cecilia Villa-Rosas, MD,(1,2) Rafael Laniado-Laborín, MD, MPH,(1,2) Lorena Oceguera-Palao, MD.(1,2)
(1) Clínica y Laboratorio de Tuberculosis, Hospital General de Tijuana, Instituto de Servicios de Salud Pública de Baja California (Isesalud). Baja California, México.
(2) Facultad de Medicina y Psicología, Universidad Autónoma de Baja California. Baja California, México
Abstract
Objective. To determine rates of drug resistance in new cases of pulmonary tuberculosis in a region with a high burden of the disease.
Materials and methods. New case suspects were referred for drug susceptibility testing.
Results. 28.9% of new cases were resistant to at least one first line drug; 3.9% had a multidrug-resistant strain, 15.6% a monoresistant strain and 9.4% a polyresistant strain.
Conclusion. Our rate of drug resistant tuberculosis in new cases is very high; this has important clinical implications, since even monoresistance can have a negative impact on the outcome of new cases treated empirically with a six month regimen.
Key words: treatment outcome; resistance amplification; isoniazid; rifampin.
Resumen
Objetivo. Determinar las tasas de resistencia a fármacos en casos nuevos de tuberculosis pulmonar en una región con alta prevalencia de farmacorresistencia.
Material y métodos. Casos nuevos de tuberculosis pulmonar referidos para cultivo y pruebas de sensibilidad a los fármacos antituberculosis de primera línea.
Resultados. 28.9% de los casos nuevos presentaban una cepa resistente al menos a un fármaco de primera línea; 3.9% eran multifarmacorresistentes, 15.6% eran monorresistentes y 9.4% polirresistentes.
Conclusión. La presente tasa de tuberculosis resistente en casos nuevos es muy elevada; esto tiene importantes implicaciones clínicas ya que aún la monorresistencia puede tener un impacto negativo sobre los resultados del tratamiento de pacientes que reciben un esquema empírico de seis meses.
Palabras clave: resultado de tratamiento; amplificación de resistencia; isoniacida; rifampicina.
Drug-resistant tuberculosis (DR-TB) poses a serious challenge to global control of TB.1 Although multidrug-resistant TB (MDR-TB) has been extensively researched, there is a gap in the literature on the management of monoresistant and polyresistant forms of tuberculosis.2 Mono- and polyresistance to first-line antituberculosis medications is an ongoing global health problem; in particular, resistance to isoniazid (INH) is very common, with a global prevalence of 10% among new cases and 28% among previously treated cases.3
Although DR-TB is usually diagnosed in previously treated patients, it can also be observed in new, previously untreated cases, due to transmission of drug-resistant strains in the community.4
In many countries new cases are diagnosed based only on microscopy, and consequently DR-TB will go undetected. Our objective was to determine the rate of drug resistance in new cases of pulmonary tuberculosis in a high TB burden region.
Materials and methods
Tijuana (population 1 559 683)5 has the highest rate of tuberculosis (40-50 cases per 105) in Mexico; an average of 1 000 cases is diagnosed annually, of which 70% are new. We asked the health centers in the city to refer new TB suspects for culture and drug susceptibility testing (DST) to the TB laboratory at the Tijuana General Hospital; due to resource limitations we cultured new cases only once a week. The study period included subjects from January 1, 2011 through June 30, 2013.
Clinical information was obtained in every case, with emphasis on ruling out a previous diagnosis and treatment of TB; every subject was tested for HIV infection. Sputum samples were processed for microscopy, liquid culture (MGIT 960Ò, Beckton Dickinson, NJ) and solid culture (Lowenstein-Jensen/Stonebrink). Positive cultures for Mycobacterium tuberculosis (MTB) were tested for drug susceptibility to first line drugs (isoniazid [INH], ethambutol [EMB], rifampin [RIF], pyrazinamide [PZA] and streptomycin [SM]) with the MGIT 960Ò system.
The study protocol was approved by the ethics committee of the hospital. Subjects were required to sign an informed consent to participate.
Results
A total of 214 subjects with clinical suspicion of tuberculosis were referred for culture. Mean age was 38.5 ± 13.9 years; 146 (67%) were males. All the subjects had had productive cough for more than two weeks. Nineteen subjects (8.8%) had a positive HIV test.
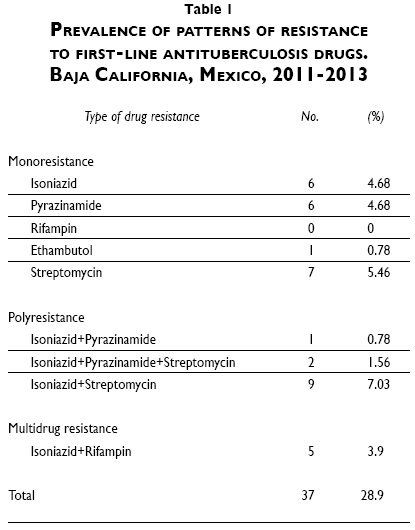
One hundred and twenty-eight patients (59.8%) had a positive culture for MTB and 37 (28.9%) had a MTB strain resistant to at least one first line drug. Twenty-three (17.9%) were resistant to INH (including monoresistant, polyresistant and MDR cases), 5 to RIF (3.9%), 14 to PZA (10.9%), 3 to EMB (2.3%) and 22 to SM (17.2%). Five patients (3.9%) had an MDR strain, 20 (15.6%) had a monoresistant strain and 12 (9.4%) had a polyresistant strain (table I). There were no significant differences in sociodemographic characteristics between subjects infected with a resistant strain and those infected with pan-susceptible mycobacteria. Ten patients with negative smears (7.8%) had a positive culture (three had a resistant strain: one resistant to SM, one resistant to PZA, and one resistant to INH and SM).
Discussion
In most developing countries DST is reserved for patients who experience treatment failure or are undergoing retreatment. In the absence of routine DST, new cases will receive a standardized regimen that includes four first line drugs.6
In contrast, treatment of drug-resistant tuberculosis often requires second-line drugs and should be guided by DST results. Unfortunately, in many countries with high rates of drug-resistant tuberculosis, new cases with undiagnosed drug-resistance are treated empirically with first-line drugs, based only on microscopy results. There are several reasons that explain the adoption of this programmatic policy, the most significant being the absence of laboratory facilities capable of performing DST and a limited access to second-line drugs.4
Standardized short-course chemotherapy with first line drugs has been shown to be less effective against drug-resistant tuberculosis than against drug-susceptible tuberculosis.4 A recent global meta-analysis among patients with INH monoresistance found failure rates ranging from 18 to 44%.7 A study conducted in Tomsk Oblast, reported that 70.8% of patients with pretreatment isoniazid- or rifampin-resistant strains (but without MDR-TB at onset), were found to have developed MDR-TB after treatment had failed.4 The optimal management of INH monoresistant TB has been widely debated; current global recommendations are that INH monoresistant TB should be treated with a 9-month regimen of daily rifampin, pyrazinamide and ethambutol (9RIF+PZA+EMB).8
Pirazinamide [PZA] has an important sterilizing effect, and significantly reduces relapse rates in patients treated with a 6-month regimen (ZINH+RIF+PZA+EMB/4INH3RIF3). Patients with monoresistance to PZA have higher rates of relapse, failure and mortality when compared with those of patients with full susceptible strains.1 Loss of PZA from the regimen requires prolonging the duration of therapy with INH and RIF by 3 months, for a total of 9 months therapy.9
Roughly, one fifth of our new cases were resistant to INH; this scenario carries a very high risk of amplification of resistance during the continuation phase; a patient with an INH resistant strain would be in monotherapy with RIF for four months and vice versa. In both cases the development of MDR-TB would be highly likely. This risk would be higher in polyresistant cases that are left with a very weak standardized regimen. Finally, a new MDR-TB case will certainly extend its resistance pattern to EMB, PZA or both.
Conclusion
Early diagnosis of drug resistant tuberculosis and the judicious use of second-line drugs are recommended to decrease transmission of drug-resistant strains and to prevent the creation of multidrug-resistant strains.4 Thirty-five years ago Stefan Grzybowski wrote: "It is far better to do nothing than to treat the cases badly".10 We agree.
References
1. Yee DP, Menzies D, Brassard P. Clinical outcomes of pyrazinamide-monoresistant Mycobacterium tuberculosis in Quebec. Int J Tuberc Lung Dis 2012;16:604-609. [ Links ]
2. Gegia M, Cohen T, Kalandadze I, Vashakidze L, Furin J. Outcomes among tuberculosis patients with isoniazid resistance in Georgia, 2007-2009. Int J Tuberc Lung Dis 2012;16:812-816. [ Links ]
3. Dantes R, Metcalfe J, Kim E, Kato-Maeda M, Hopewell PC, Kawamura M, et al. Impact of isoniazid resistance-conferring mutations on the clinical presentation of isoniazid monoresistant tuberculosis. PLoS ONE 2012;7:e37956. doi:10.1371/journal.pone.0037956. [ Links ]
4. Seung KJ, Gelmanova IE, Peremitin GG, Golubchikova VT, Pavlova VE, Sirotkina OB, et al. The effect of initial drug resistance on treatment response and acquired drug resistance during standardized short-course chemotherapy for tuberculosis. Clin Infect Dis 2004;39:1321-1328. [ Links ]
5. Instituto Nacional de Estadística, Geografía e Informática. Número de habitantes [online monograph]. México: Inegi, 2014. [accessed on 2014 December 17]. Aviable at: http://cuentame.inegi.org.mx/monografias/informacion/bc/poblacion/ [ Links ]
6. World Health Organization. Treatment of tuberculosis: guidelines-4th ed WHO/HTM/TB/2009.420. Geneva: WHO, 2009: 420. [ Links ]
7. Menzies D, Benedetti A, Paydar A, Royce S, Pai M, Burman W, et al. Standardized treatment of active tuberculosis in patients with previous treatment and/or with mono-resistance to isoniazid: a systematic review and meta-analysis. PLoS Med 2009;6:e1000150. doi:10.1371/journal.pmed.1000150. [ Links ]
8. World Health Organization. Guidelines for the programmatic management of drug-resistant TB: emergency update 2008. Geneva, Switzerland: WHO, 2008. [ Links ]
9. Curry International Tuberculosis Center and California Department of Public Health. Drug-resistant tuberculosis: a survival guide for clinicians. 2nd ed. San Francisco, California: University of California, 2011:33. [ Links ]
10. Grzybowski S, Enarson D. The fate of cases of pulmonary tuberculosis under various treatment programmes. Bull Int Union Tuberc Lung Dis 1978;53:70-75. [ Links ]
Received on: May 8, 2014
Accepted on: January 13, 2015
 Corresponding author:
Corresponding author:
Dr. Rafael Laniado Laborín.
Facultad de Medicina y Psicología,
Universidad Autónoma de Baja California.
Emiliano Zapata 1423, zona Centro.
22 000 Tijuana, Baja California, México.
E-mail: rlaniado@uabc.edu.mx
Declaration of conflict of interests. The authors declare that they have no conflict of interests.













