Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Salud Pública de México
versión impresa ISSN 0036-3634
Salud pública Méx vol.56 no.5 Cuernavaca sep./oct. 2014
Artículo original
Cost analysis of different cervical cancer screening strategies in Mexico
Análisis de costos de distintas estrategias de tamizaje para cáncer cervical en México
Christyn M Beal, MD, MPH,(1) Jorge Salmerón, MD, DSc,(1,2) Yvonne N Flores, PhD,(1,3) Leticia Torres, MSc,(1,2) Víctor Granados-García, MSc,(4) Ellen Dugan, MPH,(1) Eduardo Lazcano-Ponce, MD, DSc.(2)
(1) Unidad de Investigación Epidemiológica y en Servicios de Salud, Instituto Mexicano del Seguro Social. Cuernavaca, Morelos, México.
(2) Centro de Investigación en Salud Poblacional, Instituto Nacional de Salud Pública. Cuernavaca, Morelos, México.
(3) UCLA Department of Health Policy and Management, Fielding School of Public Health and Jonsson Comprehensive Cancer Center. Los Angeles, California, EUA.
(4) Unidad de Investigación Epidemiológica y en Sistemas de Salud, Área de Envejecimiento, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social. Distrito Federal, México.
Abstract
Objective. To compare the costs and number of undetected cases of four cervical cancer screening strategies (CCSS) in Mexico.
Materials and methods. We estimated the costs and outcomes of the following CCSS: a) conventional Papanicolaou smear (Pap) alone; b) high-risk human papilloma virus testing (HR-HPV) as primary screening with Pap as reflex triage; c) HR-HPV as primary screening with HPV-16/18 typing, liquid-based cytology (LBC) and immunostaining for p16/Ki67 testing as reflex triage, and d) co-testing with HR-HPV and LBC with HPV-16/18 typing and immunostaining for p16/Ki67 as reflex triage. The outcome of interest was high-grade cervical lesions or cervical cancer.
Results. HR-HPV testing, HPV typing, LBC testing and immunostaining is the best alternative because it is the least expensive option with an acceptable number of missed cases.
Conclusions. The opportunity costs of a poor quality CCSS is many false negatives. Combining multiple tests may be a more cost-effective way to screen for cervical cancer in Mexico.
Key words: uterine cervical neoplasms; screening; human papillomavirus DNA test; Mexico.
Resumen
Objetivo. Comparar los costos y los casos no detectados de cuatro estrategias de tamizaje de cáncer cervical (ETCC) en México.
Material y métodos. Se estimaron los costos y resultados en salud de las siguientes ETCC: a) citología convencional como único procedimiento de tamizaje; b) detección de virus del papiloma humano de alto riesgo (VPH-AR) como tamizaje primario y citología convencional como procedimiento de triage; c) detección de VPH-AR como tamizaje primario y tipificación de VPH-16/18, citología en base líquida e inmunotinción para p16/Ki67 como procedimientos de triage, y d) evaluación conjunta con VPH-AR y citología en base líquida como tamizaje primario y tipificación de VPH-16/18 e inmunotinción para p16/Ki67 como procedimientos de triage. El resultado en salud analizado fueron los casos de neoplasia intraepitelial cervical (CIN 2/3) o cáncer cervical detectados.
Resultados. La ETCC basada en la detección de VPH-AR como prueba primaria y seguida de la tipificación de VPH-16/18, la citología en base líquida y la inmunotinción para p16/Ki67 como procedimientos de triage es la mejor alternativa, ya que es la menos costosa y la que tuvo un nivel aceptable de casos perdidos.
Conclusiones. El costo de oportunidad de una ETCC de mala calidad es un alto número de falsos negativos. La combinación seriada de varias pruebas de tamizaje y triage puede ser una alternativa costo-efectiva para la detección oportuna de cáncer cervical en México.
Palabras clave: cáncer de cuello uterino; tamizaje; pruebas de ADN del papillomavirus humano; México.
Cervical cancer (CC) is a serious public health problem for women worldwide. But this is especially true for women in developing countries, where most cases occur.1 In Mexico, CC is the second leading cause of death due to cancer and over 4 000 women die from this disease each year.2-4 A significant number of these deaths could be prevented by improving existing cervical cancer screening programs (CCSP).5 Healthcare organizations have a variety of options when selecting the most appropriate cervical cancer screening strategy, but they have to take into account the needs and resources of their particular setting or context.6 For a CCSP to be effective, the screening test(s) must correctly identify potentially metaplastic lesions, and the program should be widely available and reasonably priced.7 Some of the resources that should be considered before choosing a screening strategy include: the patient population, losses due to follow-up, financial resources, personnel, and healthcare infrastructure.
For the past 50 years, the conventional Papanicolaou (Pap) smear has been the primary screening test used to detect malignant cervical lesions.8 Although the Pap smear has played a key role in the reduction of CC deaths in many countries, the mortality rate continues to be high in many Latin American countries, including Mexico.9 Pap smear-based CCSP are difficult to implement and ineffective in resource-poor settings because of the taxing infrastructure requirements including laboratory facilities, transportation and tracking systems for the specimens, trained cytopathologists, costs, and the multiple follow-up visits required.10,11 In addition to the significant healthcare resources required to provide the Pap smear, the sensitivity of this test varies greatly between countries and different healthcare facilities, making it a non-ideal screening tool.
The availability of high-risk human papilloma virus DNA (HR-HPV) testing represents a very important breakthrough for the prevention and detection of invasive CC.12 HR-HPV testing can be used alone or in conjunction with the Pap smear.13 A study conducted by Ronco and collaborators demonstrated an increased sensitivity for detecting high-grade cervical lesions when specimens are tested with cytology and HR-HPV.14 Although the cut-off points for a positive HR-HPV test can vary,15 it has become quite clear that HR-HPV testing is more sensitive than the Pap smear at detecting high-grade lesions.16 The HR-HPV test can be used in different ways, as a triage test after a positive Pap smear (Pap+/abnormal),17 or it can be used first followed by conventional (Pap smear) or liquid-based cytology as triage if the HR-HPV test is positive (HPV+). Additional possibilities include direct referral for colposcopy if the test is HPV+ or the simultaneous use of liquid-based cytology (LBC) and the HR-HPV test (co-testing). The ability to correctly identify women with disease is usually improved if the HR-HPV test is used, regardless of what triage strategy is employed.8 The result is fewer false negative results, which frequently leads to lower treatment costs in the future.8,18
Combining HR-HPV testing with LBC, specifically testing for HR-HPV types 16 and 18, and immunostaining for progression biomarkers p16/Ki67 is a novel triage option that significantly decreases false negatives. When reasonably priced, these screening and triage strategies may be cost-saving and can optimize the limited resources of healthcare institutions in less developed settings such as Mexico.
Materials and methods
Study design
A cost analysis of four different CC screening strategies was undertaken using the perspective of the major healthcare institutions of Mexico. The main outcome was the total cost of each alternative, which was defined as the screening and diagnostic costs plus the cost to treat the high-grade cervical intraepithelial neoplasia (CIN 2/3) and CC cases detected with each screening strategy. The time horizon we considered was a three-year or five-year period for screening a cohort of women. We assumed that some of these women were diagnosed with CIN 2/3 or CC as a result of the performance of the specific combination of screening tests that were used. This cost analysis focuses on the cost of implementing each screening strategy, which includes the costs of the tests used. The treatment costs concentrate on the two aforementioned outcomes (CIN 2/3 or CC) and the measure of costs is the net cost at present value using 5% discount rate and 2013 prices in US dollars (USD).
Study population
We considered a population base of 17.4 million women.19 This analysis assumes a population of 6 967 594, which corresponds to 40% of the total population of Mexican women between the ages of 35 and 64 years old covered by The Mexican Institute of Social Security (IMSS) or the Ministry of Health (SSa).19
Screening alternatives
The following four screening strategies were selected because they are currently being used by healthcare institutions or are options that will become available in the near future:
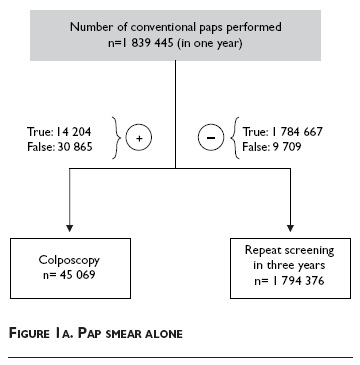
• Pap smear. Conventional Pap smear alone (figure 1a);
• HR-HPV plus Pap smear. HR-HPV testing followed by triage with the conventional Pap smear for HPV+ women (figure 1b);
• HR-HPV plus molecular triage. HR-HPV testing followed by typing for HPV-16/18, LBC triage, and immunostaining with progression biomarkers p16/ki67 for women with an abnormal LBC test (figure 1c);
• Co-testing. LBC and HR-HPV testing, followed by typing for HPV-16/18, and immunostaining for progression biomarkers p16/Ki67 in women negative for HPV types 16/18 (figure 1d).
The sensitivities and specificities for each test were obtained from previous studies,9,18,20-25 and are listed on table I. The two largest health care institutions in Mexico, IMSS and SSa, each cover approximately 6967594 women, and have a goal of screening 80% (5574075) of this population.19 To determine the number of women who should be screened in one year, this final number (5574075) was multiplied by 33% for the Pap smear alone alternative and by 20% for the other three options. This was done because the Pap smear on its own should be performed every three years, so in one year 33% of the population should be screened. In contrast, the screening interval for HR-HPV testing can be lengthened to five years, which is why 20% of the population should be screened each year with the other three alternatives. Thus, a larger number of women need to be screened each year if the Pap alone is used, as compared to the other options, which can be performed every five years.26
For the first screening alternative, the Pap smear alone, all women receive a conventional Pap smear and the Pap+ women are directly referred to colposcopy. Women with a negative Pap smear will be rescreened in three years. Figure 1a shows the total number of women who would undergo each test, as well as the expected number of true positives, true negatives, false positives, and false negatives associated with this screening strategy.
For the second option, HR-HPV plus Pap smear, all women are first tested for HR-HPV and the HR-HPV+ women are referred for triage with a conventional Pap smear. Pap+ women are referred to colposcopy and those with a normal Pap result are rescreened one year later with both HR-HPV testing and a Pap smear.16 All Pap+ women, regardless of their HPV status, are referred to colposcopy. Women with a normal Pap who remain HR-HPV+ after one year, will also be sent to colposcopy, because a persistent infection with HR-HPV is a known risk factor for CC.27 Women with a normal Pap who are HR-HPV-negative, and those who initially tested negative for HR-HPV with the first screening test will be rescreened in five years (figure 1b).
As part of the third screening alternative, HR-HPV plus molecular triage, all women are tested for HR-HPV. The HR-HPV+ women are specifically tested for HPV types 16 and 18, because 70% of CC cases are caused by these two HPV types.28 The HPV 16/18+ women are referred to colposcopy. Women who are negative for types HPV-16/18, receive LBC testing. LBC+ women are referred to colposcopy. Samples with a normal LBC test will undergo immunostaining for p16/Ki67, and the P16/Ki67+ women will also be sent to colposcopy. If all tests except for the first HR-HPV test are negative, these women will be retested in five years. If the original HR-HPV test is negative the patient will be retested in five years (figure 1c).
The fourth strategy, co-testing, simultaneously screens women using both the HR-HPV test and LBC testing. Women with both a normal LBC and negative HR-HPV test will be rescreened in five years. Women who are Pap+ and HR-HPV+ are referred for colposcopy. Women who have a normal LBC, but are HR-HPV+ will have their sample tested for HPV-16/18. Those who are HPV-16/18+ will be referred to colposcopy, and those who are negative for HPV-16/18 will be immunostained for progression biomarkers p16/Ki67. Women who are p16/Ki67+ will be sent to colposcopy. Women with a negative stain will be rescreened in five years (figure 1d).
Assumptions
The number of women in each triage arm was based on the sensitivity and specificity of each test, which were used to determine the number of true positives and true negatives respectively (table I). These estimates in conjunction with a prevalence of 13 cases per 1 000 women20 were used to calculate the false positives and false negatives. We also assumed for both true positives and false negatives that 88% would have CIN2/3, and 12% would have CC.20 The ratio between CIN2 and CIN3 was assumed to be 4:5.29 Additionally, we assumed 57% of CIN2 and 68% of CIN3 had the potential to progress to CC.30 Determining the sensitivity and specificity for the conventional Pap smear is difficult because it varies significantly depending on the study or country. This variation is largely due to the subjective interpretation of the Pap smear by the cytopathologists, and whether the sample collected was adequate for reading.31 Additionally, the repetitive nature of slide reading creates a large opportunity for interpretive errors.31 For this analysis we used a sensitivity of 59.4% and a specificity of 98.3% for the Pap smear, based on previously reported data from Mexico.20 For the HR-HPV plus Pap smear alternative we used a sensitivity of 53.8% for the Pap, based on a study that examined the sensitivity of the Pap smear when used only on HPV+ women.22 The specificity in that same study was 99.1%.22 All other sensitivity and specificity estimates were chosen based on the studies listed in table I.9,18,20-25
Several assumptions were made about the outcomes of each arm in the flow diagrams. We assumed that 100% of those referred to colposcopy would receive a biopsy meaning lost to follow-up would be 0%. The individual costs for each procedure and treatment were based on the prices reported on table I. The Pap cost including staff, supply, capital and overhead is 14.04 USD. For a Pap smear with HR-HPV testing the price of the Pap is unchanged, and the price of the HR-HPV test is 29.91 USD. Co-testing is estimated to cost 43.25 USD, and HR-HPV plus molecular triage would package all of the tests together and cost 41.04 USD. The cost of a colposcopy with biopsy is 69.01 USD. The cost of treating a case of CIN2/3 is 2 099 USD, and the cost to treat CC is 8 974 USD (table I).
Cost-effectiveness analysis
Our findings are reported as base case results that consider previously estimated values of sensitivity, specificity, costs of screening tests and treatments. The average cost effectiveness ratio was estimated considering the cost of the alternative divided by the total number of missed cases of CIN2/3 or CC avoided. The positive outcome of missed cases avoided was estimated by substracting the number of missed cases of CIN2/3 or CC from a constant of 10 000. The incremental cost per case avoided was estimated by considering the additional cost and the additional missed cases avoided by each alternative as compared with Pap alone option, which was the comparator for all strategies.
Sensitivity analysis
A probabilistic sensitivity analysis (PSA) was conducted to explore the effect of different parameter values on the base case results, which are reported on table I. The sensitivity and specificity values for the PSA were based on previously published results and the range in values for the costs was assumed to be ±30%. In order to conduct the PSA we assumed a gamma inverse distribution for costs and a normal distribution for the test sensitivity and specificity parameters. A Monte Carlo simulation of 1 000 iterations of the joint distribution of all parameters was conducted. The results of the simulation are reported considering three cost-effectiveness acceptability curves (CEAC) that account for a willingness-to-pay range from 0 to 3 000 USD. The CEAC of all alternatives are reported in a graph using the same scale for the y and x axes for comparability and space restrictions (figure 2).
Results
Our findings indicate that the final total cost of each option is 98.8 million USD for Pap smear alone, 97.8 million USD for HR-HPV plus Pap smear, 91.5 million USD for HR-HPV plus molecular triage, and 93.9 million USD for co-testing. For all strategies, the cost to treat cases of CIN2/3 and CC is the greatest expense. The treatment costs for the Pap smear alone option is approximately 69.9 million USD, while the HR-HPV plus Pap smear, HR-HPV plus molecular testing, and co-testing spend 39.5 million USD, 42.3 million USD, and 41.5 million USD, respectively. Another important factor to consider is the number of cases missed by each screening strategy. The Pap smear alone misses 9 079 cases, while the HR-HPV plus Pap smear misses 630 cases. The HR-HPV plus molecular triage, and co-testing strategies miss 717 and 546 cases, respectively (table II).
The base case analysis results regarding the cost per additional missed case prevented suggest that HR-HPV plus molecular triage and co-testing are the dominant strategies because they are less expensive and have less missed cases than the Pap alone (table III).
Sensitivity analysis
The results of the probabilistic sensitivity analysis (PSA) suggest that the alternative with the best performance as measured in terms of the cost per additional missed case prevented are the HR-HPV plus molecular triage option followed by the co-testing alternative (figure 2). These two alternatives are less expensive and have less missed cases of CIN 2/3 and CC.
The results of the Monte Carlo simulation suggest that the probability of being cost effective of the alternative HPV plus molecular triage alternative vs. Pap alone is 90% when the WTP is about 1 200 USD. The probabilities of being cost effective in the case of co-testing vs. Pap alone, and HR-HPV plus Pap vs Pap alone, are a corresponding WTP of 1 500 and 2 400 USD. The alternative of Pap alone is not cost effective in any of the pairwise comparisons conducted in the PSA, because it does not reach 80 or 90% of iterations even at very low values of cost per missed case prevented (0 to 600 USD).
Discussion
Our results suggest that the HR-HPV plus molecular triage strategy is the lowest cost option at 91.5 million USD. However, HR-HPV plus molecular triage misses 171 more cases than co-testing, and 1.7 million USD more must be spent in order to find these extra cases with the co-testing strategy. The Pap smear alone is the most expensive option and has the highest number of missed cases. The difference in cost between the Pap smear alone and HR-HPV plus molecular triage is 7.3 million USD. However, HR-HPV plus molecular triage identifies almost 9 000 more cases of CIN2/3 or CC than the Pap smear alone.
HR-HPV plus molecular triage saves 6.3 million USD when compared to HR-HPV plus Pap smear. The additional funds for HR-HPV plus Pap smear can largely be attributed to the cost of retesting over 105 000 women. Both options spend roughly the same amount on HPV testing and colposcopies. HR-HPV plus molecular triage sends 5 000 fewer women to colposcopy and performs about 3 000 fewer unnecessary colposcopies. In Mexico, colposcopies are expensive and not offered in many locations, so it is important to limit any unnecessary procedures.32
CCSPs with a low sensitivity put women at higher risk of developing invasive disease, if it is not detected at an early stage, and this directly translates into higher costs.33 The Pap smear alone option has the highest number of missed cases of CIN2/3 and CC (n= 9 709) followed by the HR-HPV plus Pap smear (n= 630), the HR-HPV plus molecular triage (n= 717), and co-testing (n= 546). This same trend is observed when comparing the treatment costs across all four options. The Pap smear alone, with the lowest sensitivity, costs approximately 40% more due to treatment expenses because it misses more cases than the other three options combined. Although, the HR-HPV plus molecular triage alternative appears to most optimally use limited healthcare dollars it must be supported by infrastructure and an efficient healthcare system in order to reduce the morbidity of CC. And, in the long term, early detection may reduce CC mortality because the risk of cancer is decreased.34 Furthermore, the costs estimated in this model will likely be modified dramatically when young girls vaccinated against HPV become old enough to be screened. Although CC screening will be required for this group as well, the screening interval can be significantly lengthened.
The estimates for each CC screening strategy were based on price estimates from a previous analysis.18,25 Based on these estimates HR-HPV plus molecular triage is the lowest cost CC screening strategy. It is important to mention that this model assumes 0% of patients will be lost to follow-up, but based on a previous study the lost to follow-up rate is approximately 37%.20 This rate would likely translate into an increased number of missed cases and higher treatment costs. Because there will inevitably be cases lost to follow-up, it is important to identify as many cases as possible by maximizing the sensitivity of the initial screening test.16 Additionally, this model assumes 100% treatment success, i.e. none of the treated cases discovered in one year of the screening program appear as a case in the next year of screening. This is not likely to be true. Also, because the HR-HPV plus molecular triage option is relatively new, additional studies like the one conducted by Denny and collaborators in South Africa must be undertaken to examine how it performs in a real life setting.32
This cost analysis contributes to the existing body of knowledge on CC screening by comparing different screening alternatives. Additionally, it provides further evidence of the advantages of using the HR-HPV test as the initial screening modality. The potential benefits of a CCSP that uses HR-HPV testing as the primary screening tool have been demonstrated in both epidemiological studies and cross-sectional comparisons.16 However, large trials are still needed to assess the impact of using HR-HPV testing on CC incidence and mortality. Moreover, as women and young girls who are vaccinated against HPV become eligible for CC screening, further investigation into the utility of HR-HPV testing will be warranted. Screening programs will likely need to undergo additional modifications to accommodate the vaccinated population.
References
1. Program for Appropriate Technology in Health. National history of cervical cancer: even infrequent screening of older women saves lives. Cervical Cancer Prevention Fact Sheet [internet series]. Seattle: Path, 2000. [Accessed September 17, 2014]. Available at: http://www.path.org/publications/files/RH_natural_history_of_cc_fs.pdf [ Links ]
2. Palacio-Mejía LS, Lazcano-Ponce E, Allen-Leigh B, Hernández-Ávila M. Regional differences in breast and cervical cancer mortality in Mexico between 1979-2006. Salud Publica Mex 2009;51(2):S208-S219. [ Links ]
3. Hernández-ávila M, Lazcano-Ponce EC, Alonso-de Ruiz P, Romieu I. Evaluation of the cervical cancer screening program in Mexico: a population-based case-control study. Int J Epidemiol 1998;27:1-7. [ Links ]
4. Lazcano-Ponce EC, Rascón-Pacheco RA, Lozano-Ascencio R, Velasco-Mondragón HE. Mortality from cervical carcinoma in Mexico: impact of screening, 1980-1990. Acta Cytol 1996;40:506-512. [ Links ]
5. America Society of Clinical Oncology. Cervical cancer screening with vinegar could prevent thousands of death each year in developing countries [internet series]. 2013. [Accessed September 17, 2014]. Available at: http://www.cancer.net/research-and-advocacy/research-summaries/cervical-cancer-screening-vinegar-could-prevent-thousands-deaths-each-year-developing-countries [ Links ]
6. Gravitt P, Coutlee F, Iftner T, Sellors J, Quint W, Wheeler C. New technologies in cervical cancer screening. Vaccine 2008;26S:K42-K52. [ Links ]
7. Placide J, Martens MG. Comparing screening methods for osteoporosis. Curr Womens Health Rep 2003;3(3):207-210. [ Links ]
8. Waxman A. Guidelines for cervical cancer screening: history and scientific rationale. Clin Obstet Gynecol 2005;48(1):77-97. [ Links ]
9. Yeoh G, Tse M, Chan K, Lord L. Human papillomavirus DNA and liquid-based cervical cytology cotesting in screening and follow-up patient groups. Acta Cytol 2006;50(6):627-631. [ Links ]
10. Huchko M, Sneden J, Leslie H, Abdulrahim N, Maloba M, Bukusi E, Cohen C. A comparison of two visual inspection methods for cervical cancer screening among HIV-infected women in Kenya. Bull World Health Organ 2014;92(3):195-203. [ Links ]
11. Flores Y, Shah K, Lazcano E, Hernández M, Bishai D, Ferris D, et al. Design and methods of the evaluation of an HPV-based cervical cancer screening strategy in Mexico: The Morelos HPV Study. Salud Publica Mex 2002;44(4):335-344. [ Links ]
12. Bosch F, Qiao Y, Castellsagué X. The epidemiology of human papillomavirus infection and its association with cervical cancer. Int J Gynaecol Obstet 2006;94:S8-S21. [ Links ]
13. Luu H, Dahlstrom K, Mullen P, VonVille H, Scheurer M. Comparison of the accuracy of Hybrid Capture II and polymerase chain reaction in detecting clinically important cervical dysplasia: a systematic review and meta-analysis. Cancer Med 2013;12(3):367-390. [ Links ]
14. Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Palma P, Mistro A, et al. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst 2008;100(7):492-501. [ Links ]
15. Schiffman M, Herrero R, Hildesheim A, Sherman M, Bratti M, Wacholder S, et al. HPV DNA testing in cervical cancer screening results from women in a high-risk province in Costa Rica. JAMA 2000;283(1):87-93. [ Links ]
16. Cuzick J, Mayrand M, Ronco G, Snijders P, Wardle J. Chapter 10: New dimensions in cervical cancer screening. Vaccine 2006;24 suppl 3:S3/S90-S97. [ Links ]
17. Arbyn M, Sasieni P, Meijer C, Clavel C, Koliopoulos G, Dillner J. Chapter 9: Clinical applications of HPV testing: A summary of meta-analyses. Vaccine 2006;24 suppl 3:S3/S78-S89. [ Links ]
18. Flores Y, Bishai D, Lörincz A, Shah K, Lazcano-Ponce E, Hernández M, et al. HPV testing for cervical cancer screening appears more cost-effective than Papanicolau cytology in Mexico. Cancer Causes Control 2011;22(2):261-272 [ Links ]
19. Instituto Nacional de Estadística y Geografía. Population: distribution by age and sex [internet series]. México: Inegi, 2010. [Accessed September 17, 2014]. Available at: http://www3.inegi.org.mx/Sistemas/temas/Default.aspx?s=est&c=17484 [ Links ]
20. Salmerón J, Lazcano-Ponce E, Lorincz A, Hernández M, Hernández P, Leyva A, et al. Comparison of HPV-based assays with Papanicolaou smears for cervical cancer screening in Morelos State, Mexico. Cancer Causes Control 2003;14(6):505-512. [ Links ]
21. Cuzick J, Clavel C, Petry K, Meijer C, Hoyer H, Ratnum S, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006;119:1095-1101. [ Links ]
22. Mayrand M, Duarte-Franco E, Rodrigues I, Walter S, Hanley J, Ferenczy A, et al. Human papullomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007;357(16):1579-1588. [ Links ]
23. Einstein M, Martens M, Garcia F, Ferris D, Mitchell A, Day S, Olson M. Clinical validation of the Cervista HOV HR and 16/18 genotyping tests for use in women with ASC-US cytology. Gynecol Oncol 2010;118:116-122. [ Links ]
24. Zappacosta R, Colasante A, Viola P, D'Antuono T, Lattanzio G, Capanna S, et al. Chromogenic in situ hybridization and p16/Ki67 dual staining on formalin-fixed paraffin-embedded cervical specimens: correlation with HPV-DNA test, E6/E7 mRNA test, and potential clinical applications. Biomed Res Int 2013;2013: 453606. [ Links ]
25. Insinga RP, Dasbach EJ, Elbasha EH, Puig A, Reynales-Shigematsu LM. Cost-effectiveness of quadrivalent human papillomavirus (HPV) vaccination in Mexico: a transmission dynamic model-based evaluation. Vaccine 2007;26(1):128-39. [ Links ]
26. Moyer VA, US Preventive Services Task Force. Screening for Cervical Cancer: U.S. Preventive Services Task Force recommendation statement [internet series]. Ann Intern Med 2012;156(12):880-891. [Accessed September 17, 2014]. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/cervcancer/cervcancerrs.pdf [ Links ]
27. Bernal-Silva S, Granados J, Gorodezky C, Aláez C, Flores-Aguilar H, Cerda-Flores RM, et al. HLA-DRB1 Class II antigen level alleles are associated with persistent HPV infection in Mexican women; a pilot study. Infect Agent Cancer 2013;8(1):31. [ Links ]
28. Bian M, Cheng J, Ma L, Cong X, Liu J, Chen Y, Chen X. Evaluation of the detection of 14 high-risk human papillomaviruses with HPV 16 and HPV 18 genotyping for cervical cancer screening. Exp Ther Med 2013;6(5):1332-1336. [ Links ]
29. Lazcano-Ponce E, Lörinz A, Salmerón J, Fernández I, Cruz A, Hernández P, et al. A pilot study of HPV DNA and cytology testing in 50 159 women in the routine Mexican Social Security Program. Cancer Causes Control 2010;21:1693-1700. [ Links ]
30. Duggan M, Brasher P. Accuracy of Pap test reported as CIN I. Diagn Cytopathol 1999;21(2):129-136. [ Links ]
31. Spitzer M, Apgar B, Brotzman G. Management of histologic abnormalities of the cervix. Am Fam Physician 2006;73(1):105-112. [ Links ]
32. Denny L, Kuhn L, Pollack A, Wainwright H, Wright T. Evaluation of alternative methods of cervical cancer screening for resources-poor settings. Cancer 2000;89(4):826-833. [ Links ]
33. Koliopoulos G, Arbyn M, Martin-Hirsch P, Kyrgiou M, Prendiville W, Paraskevaidis E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and meta-analysis of non-randomized studies. Gynecol Oncol 2007;104:232-246. [ Links ]
34. Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand M, et al. Overview of human pappillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine 2008;19:26 suppl 10:K29-K41. [ Links ]
Received on: March 18, 2014
Accepted on: August 7, 2014
 Corresponding author:
Corresponding author:
Dr. Jorge Salmerón.
Unidad de Investigación Epidemiológica y en Servicios de Salud,
Instituto Mexicano del Seguro Social. Blvd. Benito Juárez 31
col. Centro. 62000 Cuernavaca, Morelos, México.
E-mail: jorge.salmec@gmail.com
Declaration of conflict of interests: The authors declare not to have conflict of interests.