Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Salud Pública de México
versión impresa ISSN 0036-3634
Salud pública Méx vol.56 no.2 Cuernavaca mar./abr. 2014
Artículo original
ATM polymorphisms IVS24-9delT, IVS38-8T>C, and 5557G>A in Mexican women with familial and/or early-onset breast cancer
Polimorfismos IVS24-9delT, IVS38-8T>C y 5557G>A en el gen ATM en mujeres mexicanas con cáncer de mama familiar o de inicio temprano
Fabiola del Carmen Calderón-Zúñiga, M en C,(1) Guadalupe Ocampo-Gómez, M en C,(1) Francisco Carlos López-Márquez, D en C,(1) Rogelio Recio-Vega, D en C,(1) Luis Benjamín Serrano-Gallardo, D en C,(1) Pablo Ruiz-Flores, D en C.(1)
(1) Centro de Investigación Biomédica, Universidad Autónoma de Coahuila. Torreón, Coahuila, México.
Abstract
Objective. To assess whether in Mexican population the frequencies of ATM polymorphisms IVS24-9delT, IVS38-8-T>C, and 5557G>A in breast cancer (BC) cases and healthy controls were different from those found in other countries.
Materials and methods. Frequencies of polymorphisms conferring BC risk IVS24-9delT, IVS38-8T>C, and 5557G>A were analyzed by PCR-RFLP in 94 patients with familial and/or early onset BC, and 97 healthy controls randomly selected. Allele frequencies analysis was done using χ2 and Hardy-Weinberg test.
Results. Frequencies of heterozygous were: for 5557G>A, 13% cases, 0%controls (p=0.0009); for IVS24-9delT, 21% cases, 8% controls (p=0.0122); for IVS38-8T>C, only one case. 5557G>A and IVS24-9delT were more frequent in cases than in controls. The allelic frequencies found in 5557G>A are similar to those described by González-Hormazábal in Chile.
Conclusion. The similarity of results in this polymorphism between Chilean and Mexican populations may be due to both being crossbred with an Amerindian-Spanish component, while differences may be due to fact that Chilean population has a greater European component than Mexican's.
Key words: ataxia telangiectasia mutated (ATM); breast cancer; single nucleotide polymorphisms (SNP).
Resumen
Objetivo. Evaluar si en la población mexicana las frecuencias de los polimorfismos IVS-9delT, IVS38-8T>C y 5557G>A en casos de cáncer de mama y en controles sanos son diferentes de las encontradas en otros países.
Material y métodos. Los polimorfismos IVS24-9delT, IVS38-8T>C y 5557G>A fueron analizados mediante PCR-RFLPs en 94 pacientes con CM de tipo familiar o de inicio temprano y 97 testigos seleccionadas de forma aleatoria. El análisis de la frecuencia alélica se hizo mediante χ2 y equilibrio de Hardy-Weinberg.
Resultados. Las frecuencias de heterocigotos fueron 5557G>A, 13% de casos, 0% de testigos (p=0.0009); IVS24-9delT, 21% de casos, 8% de testigos (p=0.0l22); IVS38-8T>C, sólo un caso. 5557G>A y IVS24-9delT fueron más frecuentes en casos que en testigos. Las frecuencias alélicas encontradas en 5557G>A son similares a las descritas por González-Hormazábal en Chile.
Conclusión. La similitud de resultados en este polimorfismo entre la población chilena y mexicana puede ser debida a que ambas son mestizas con un componente amerindio-español. Las diferencias encontradas podrían explicarse porque la población chilena tiene mayor componente europeo que la mexicana.
Palabras clave: gen ataxia telangiectasia mutado (ATM); cáncer de mama; polimorfismos de nucléotido simple (PNS).
Breast cancer (BC) is a major public health problem. Worldwide, and in Latin America, BC in women between 20 and 59 years of age is the leading cause of mortality.1 Worldwide and every year, >1 million cases of BC are diagnosed and 548 000 women die due to this disease.2 For women in Mexico, BC ranks as the leading cause of death due to malignant tumors, with 5217 deaths per year.1 Early detection reduces mortality; thus, timely access to preventive health services is a key step for successful control. However, unlike what occurs in developed countries, in which 50% of cases are identified early, in Mexico nearly 90% of cases are diagnosed at later stages.3
Molecular genetic studies have discovered genes whose functional impairment is associated with an increased risk for hereditary cancer. This constitutes 5-10% of all cases of BC.4 This predisposition for BC has been well established for patients with germline mutations in BRCA1 or BRCA2 genes, whose risk of developing the disease is between 60 and 80%.5 Instead, mutations in genes of low penetrance, such as the ataxia telangiectasia mutated gene (ATM) and others, confer a lower risk, but account for a larger proportion of total cases.6
ATM is a low-penetrance gene frequently involved in hereditary susceptibility to BC.7 The ATM-kinase encoded by the ATM gene plays an essential role in the maintenance of genomic integrity through its function as an activator of the cellular response for repairing double-strand breaks in DNA. Functional loss of both alleles of the gene results in a severe inherited disorder: Ataxia-telangiectasia (AT), which is an autosomal recessive disease in which individuals typically show progressive neuronal degeneration, immunodeficiency, chromosomal instability, radio-sensitivity, and increased risk for cancer.8,9
It is estimated that approximately 0.5-1% of the general population is heterozygous for a germline mutation in the ATM gene, and heterozygous females have a relative risk (RR) of 4.9 for developing BC compared with the general population.6 Different ATM gene polymorphisms have been proposed as responsible for conferring a greater risk of developing BC. Three of these, which are most often studied, are 9delT-IVS24, IVS38-8T>C, and 5557G>A.10 It has been reported that female carriers of these variants have a higher occurrence of bilateral BC, early disease onset, radio-sensitivity, and long survival rates.11,12 In contrast, other studies have failed to find this association.13
Studies of polymorphism 5557G>A
The polymorphism 5557G>A (D1853N), located in exon 39 of the ATM gene, has been studied by Mehdipour and colleagues,14 who proposed it as a predisposing factor for developing BC, especially in familial cases. The study was conducted in an Iranian population of women with BC and in a healthy control group divided into two subgroups, one randomly selected and the other with a family history, reporting a carrier frequency of 31% in the disease group and 18.6% in controls. In the randomly formed group, carrier frequency was 12.5%, whereas in those with a positive familial history for BC, the rate was 26.9%. Gao and colleagues conducted a meta-analysis of nine epidemiological studies with 4 191 cases and 3 780 controls, concluding that there was no association between 5557G>A and the disease.15 In contrast, studies such as that of Tapia and colleagues16 found a frankly positive association between 5557G>A and the risk for developing bilateral BC in a population of Chilean women with hereditary BC. They analyzed the frequency of mutation of 5557G>A alone and in combination with the intronic variant IVS24-9delT. The results showed a 20.3% frequency of heterozygous in cases and of 7.5% in controls, while the frequency of homozygotes was only 1% in both groups. Schrauder and colleagues analyzed this variant by real time PCR, in 514 patients with BC and in 511 age-matched healthy controls, resulting in the association of this variant with the risk of developing BC (p = 0.04).17
In European population, this variant has been described by Angéle and colleagues.18 They found a higher frequency of homozygotes in cases of individuals with BC that received radiotherapy compared with those who did not (odds ratio [OR] 6.76), associating this variant with increased radio-sensitivity in breast tissue, and suggesting it as a risk factor that predisposes for reaction effects after radiotherapy. González-Hormazábal and colleagues found variants in the ATM gene in a study of 137 Chilean patients with familial BC negative for mutations in BRCA1/2, in which they analyzed polymorphisms IVS24-9delT, IVS38-8T>C, and 5557G>A (the latter more common, with 20.6%), and concluded that these three genotypes alone or in combination plus environmental factors may alter the risk for BC through increasing genetic instability or by altering the DNA damage response.10 This study was, to our knowledge, the first indication of the importance of this mutation in Latin American population.
Studies of variant IVS24-9delT
The intronic variant IVS24-9delT, located in intron 24 in the ATM gene has been associated with an increased risk of developing BC; Heikkinen and colleagues19 found a frequency of 38.0% for the mutated variant in 121 patients with familial BC or ovarian cancer. On the other hand, Tapia and colleagues16 analyzed 94 patients with BC who belonged to 78 families at high risk but who did not have mutations in BRCA1/2 and 200 healthy controls. The authors found that IVS24-9delT together with 5557G<A increased the risk for developing BC (OR, 3.97; p=0.0003). They concluded that carrying this haplotype confers an increased risk for developing BC.
In Chilean population González-Hormazábal and colleagues10studied variants 5557G>A, IVS24-9delT, and IVS38-8T>C in 126 cases negative for mutations in BRCA1/2 and in 200 controls, resulting in a 20.6% frequency of heterozygotes in cases and 13% in controls (p=0.048) for the variant IVS24-9delT. Furthermore, the combination of the three variants showed statistical significance (p=0.024) as a risk factor. The authors also concluded that this variant alone or in combination increases the risk of developing BC.
Studies in intronic variant IVS38-8T>C
It has been postulated that IVS38-8T>C confers an increased risk for developing BC. This variant is located in intron 38 of the ATM gene and has been studied in different populations with divergent results concerning its association or not with BC. For instance, in Southern Finland,20 in a study of two groups of cases, one comprising 786 women with familial BC, the other with 818 patients not selected for familial history, and 708 healthy controls, analyzed this variant and the polymorphism 5557G>A. The result was that neither of these two variants was significantly associated with the risk for BC.
Materials and methods
We analyzed a total of 94 patients with familial and/or early-onset BC who were recruited from the Department of Oncology, Clinic 71, of the Mexican Social Security Institute (Instituto Mexicano del Seguro Social, IMSS) in Torreon, Coahuila, Mexico, through years 2005-2010. Inclusion criteria: patients with histological diagnosis of breast cancer, who had at least two cases in first-degree relatives, or at least four cases in second-degree relatives, or who developed cancer at age 35 or earlier, and voluntarily accepted to participate in the study. The control group was made up of 97 women without neoplasm who were recruited from the same clinic; the latter were relatives of patients unrelated to the cases, presenting at medical services different from the Department of Oncology.
This study was approved by the Bioethics Committee of the Faculty of Medicine at the Universidad Autónoma de Coahuila in Mexico. All women participating in this study were asked to sign a letter of informed consent and to voluntarily donate 5 ml of peripheral venous blood.
Mutation analysis
Genomic DNA was extracted from peripheral blood lymphocytes. ATM gene sequences containing the allelic variants IVS24-9delT, IVS38-8T>C, and 5557G>A were amplified by PCR using primers described previously by González-Hormazábal and colleagues.14 RFLP were obtained through digestion of the amplified products using restriction enzymes FnuHI and RsaI (New England Biolabs, MA, USA) during 12 h, according to the manufacturer's specifications. These were identified by 30% polyacrylamide gel electrophoresis and silver nitrate staining, and were vacuum dried.
Statistical analysis
Allele frequencies were calculated by Hardy-Weinberg equilibrium. Differences between allele frequencies found in cases and controls were established through the chi square or Fisher test with a significance level of 0.05. These tests and OR were calculated using the STATA .ver. 10 statistical software program.
Results
Table I illustrates the frequencies of IVS24-9delT, IVS38-8T>C, and 5557G>A obtained in this study. For variant IVS24-9delT, the frequency of wild-type homozygous T/T was higher in controls (71%) than in cases (61%), while the frequency of heterozygous T/-T was higher in cases (18%) than in controls (8%), the difference being statistically significant, although it is noteworthy that the frequency of homozygotes -T/-T was similar in cases and controls (21%). The variant IVS38-8T>C showed only one case (1%) in heterozygous state, whereas the controls did not show this. Finally, for polymorphism 5557G>A, the frequency of heterozygous G/A was 13% in cases and 0% in controls. Neither cases nor controls showed mutated homozygous state A/A. Differences in frequencies between cases and controls for this polymorphism were statistically significant.
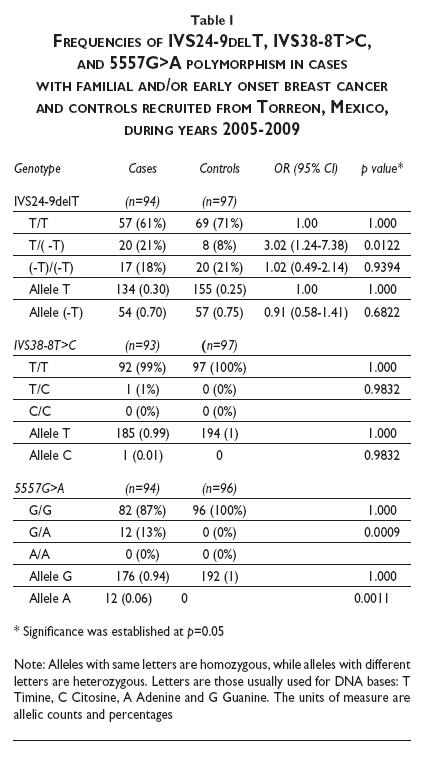
In addition to analyzing the genotypes separately, analysis was also performed on different combinations of the three polymorphisms (table II). As shown in table II, for the composed genotype IVS24-9delT/5557G>A, allelic combinations T/T, G/G, T/T, G/A, and (-G)/ (-T) G/G were statistically significant, with variants appearing more frequently in the case group. On the other hand, in the composed genotype IVS38-8T>C / 5557G>A, the allelic combinations T/T, G/A were found only in cases, with a frequency of 13% (p=0.001).

The composed genotype IVS38-8T>C/IVS24-9delT showed significant difference for the allelic combination T/(-T), T/T (22% in cases vs. 8% in controls). Finally, as expected, when the combination of the three wild-type genotypes was analyzed, frequency was significantly higher in controls than in cases. Moreover, differences were found for the combination T/T, TT, G/A, while the combination T/T, T/(-T), G/G was not significant.
Discussion
Considering the differences found between populations of various countries for the three polymorphisms described, we considered it important to ascertain whether in Mexican population the frequencies of these polymorphisms in BC cases and healthy controls were different from those found in other countries, and whether they were similar to the results from Chile, a country with whom we share a Spanish-Amerindian origin. In the analysis of clinical variables between cases and controls, there was a clear difference of 14 years in the average age between the groups, because they were not matched for age (mean, 27.75 in cases vs. 41.84 in controls; p=0.0001). This raises the possibility that over the course of 14 years, some controls may become cases. However, although the absolute risk of a woman developing BC during her lifetime is 1 in 8, the calculated risk by decade is much smaller. It has been estimated that for women up to age 30 years, the risk of BC is 1 in 233, while for women in the 40-49-years-of-age decade, the risk has been estimated at 1.4%.21 This means that according to the difference in age and the sample size of this study, it is likely that one or two of the controls will develop BC when they reach the average age of the case group, which would not significantly alter this study's results. To prove this, we matched by age the early-onset group with the control group; no variation in allele frequencies were found regarding the total cases (data not shown).
The largest difference between cases and controls was a positive familial history of BC, this being much higher in cases than in controls, which confirms the importance of genetic factors in the genesis of the disease, although familial history was a selection criterion in cases, which constitutes a selection bias.
Regarding the analysis of allele frequencies of the three polymorphisms, the study of Dork and colleagues,22 carried out in German population, for establishing the relationship between the variant 5557G>A and BC, showed 23.5% of heterozygotes in cases and 14.8% in controls, which was a statistically significant difference. Similar results were found by Mehdipour and colleagues14 in Iranian population, in which the authors found a heterozygote frequency of 31% in cases vs. 18.6% in controls. These results differ significantly from those obtained in this study, probably because they are populations with different genetic background. On the other hand, Angéle 18 found no significant difference in the frequency of allele 5557G>A between cases and controls in French population.
In a study in the US in a mixed population of different ethnic groups (including Latino population), the authors found similar frequencies in both cases and controls for mutation 5557G>A.23 They found the highest frequency of the mutation in the Caucasian group (24.5 and 21.2% in cases and controls, respectively), followed by Latino population with a frequency of 13.8% in cases and 14.3% in controls. This reported frequency in Latinos is very similar to that found in the present study (13%), which probably could be explained in two ways: 1) because the majority of the Latino cases analyzed were of Mexican origin, or 2) because Mexican population shares very similar genetic characteristics with other Latino populations. In the same study, African-American and Asian groups had lower allele frequencies: African-Americans 8.4 and 7.8% in cases and controls, respectively, and Asians, 2.3% in cases and 5.9% in controls.23
Consistent with the frequency of polymorphism 5557G>A found in the Caucasian group by Bretsky and colleagues,23 Schrauder and colleagues17 reported, in German population, a frequency of 19.3% in the case group and of 25.3% in controls.
Differences among studies, including this one, could be attributed to ethnic differences between populations. For example, in this study, the A allele is less frequent than in European population. The low frequency of this allele would explain why we found a zero frequency of homozygotes and a low number of heterozygotes.
In Chilean population, a significant difference between cases and controls for the mutation 5557G>A (p= 0.008) has been reported,16 attributing a positive association between that variant and the risk for BC, while the authors found no association between intronic variants IVS24-9delT and IVS38-8T>C, and the risk for BC. However, they found an increased risk for BC for haplotype 5557G>A/IVS24-9delT (p=0.0003), indicating that probably carriers of both polymorphisms increase the risk for BC, although 5557G>A remained as the haplotype with the greatest weight in statistical significance. In our study the composed haplotype 5557G>A/IVS24-9delT appear to confer a 3.27 fold increase in BC risk.
On analyzing IVS24-9delT, IVS38-8T>C, and 5557G>A variants in Chilean population, González-Hormazábal and colleagues reported statistical significance for all three, indicating that the presence of these variants confers an increased risk of developing BC.10 For polymorphism 5557G>A, the authors described a heterozygous frequency of 20.6% in cases and of 13% in controls. Similar frequencies were found for the variant IVS24-9delT. Moreover, they obtained frequencies of 8.7 and 3.0% between cases and controls, respectively, for the variant IVS38-8T>C. Only the heterozygous frequency of variant IVS24-9delT (20.6%) was similar to that described in our population (21%), conferring an increased risk among women with BC both in Chilean (OR 1.74) and Mexican populations (OR 3.02). Other variants show that although some results of this study are similar to those of Chile, populations are not identical.
The frequency of heterozygocity for the variant 5557G>A in this study is similar to that of previous studies in Chile. This suggests that in our population, being heterozygous for this variant also increases the risk for BC. In the case of polymorphism IVS24-9delT, although the frequency of heterozygotes in this study was higher in cases than in controls (p=0.0122) when analysis was performed by counting-T alleles in both groups, there was no significant difference. It is noteworthy that although there is a greater number of heterozygotes in cases, the frequency of homozygotes (-T) / (-T) was equal in cases and controls (21%), because when the frequency of homozygotes is the same in two populations, it is expected that the frequency of heterozygotes should also be similar.
Regarding the combination of the three variants, González-Hormazábal and colleagues obtained statistical significance for genotypes IVS24-9delT/IVS38-8T>C (p=0.021), IVS38-8T>C/5557G>A (p=0.021), IVS24-9-delT/5557G>A (p=0.048) and the combination of the three polymorphisms (p=0.021). All of these were in heterozygous state, very similar to the results found in this study, indicating that being the carrier of one of these alleles alone or in combination may alter the risk for cancer, probably through increased genetic instability or through altering the normal response for DNA damage repair, as described previously.10 Additionally, we found that haplotype T/(-T), TT, G/G appear to confer risk to BC (OR 3.41; CI 1.38-8.43); however, as can be seen in table I, in this case such risk should be attributable in fact to allele IVS24-9delT (T/-T).
It is clear that results in Chilean population are in some respects similar to those reported in this study for the three described polymorphisms. This similarity between Mexican and Chilean population may be due to the fact that both are mestizo populations, with a strong Amerindian and Spanish background. However, other frequencies are not so similar. This could be because notwithstanding the Spanish Amerindian component, it is also likely that Chilean population has a greater European component24 than Mexican population.25 The results of this study, supported by previous studies, suggest that polymorphism 5557G>A in Mexican population in particular, and perhaps in Latin American population in general, confers an increase in the risk for breast cancer.
Acknowledgments
The authors wish to thank Dr. Perla Karina Espino Silva and MC Jhazel Hernández Ávila for their valuable contribution to the analysis of samples in the laboratory. This project was funded in part by Programa Integral de Fortalecimiento Institucional (PIFI) of the Autonomous University of Coahuila.
References
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 10. Lyon, France: International Agency for Research on Cancer. [Accessed June, 2010]. Available at: http://globocan.iarc.fr [ Links ]
2. World Health Organization. WHO Fact Sheet 297: Cancer. Geneva: WHO, 2008. [ Links ]
3. Secretaría de Salud. Información en Salud, 2008. [Accessed June, 2010] Available at: http://sinais.salud.gob.mx/descargas/pdf/IB_2006.pdf. [ Links ]
4. Kuusisto KM, Bebel A, Vihinen M, Schleutker J, Sallinen SL. Screening for BRCA1, BRCA2, CHEK2, PALB2, BRIPl, RAD50, and CDH1 mutations in high-risk Finnish BRCA1/2-founder mutation-negative breast and/or ovarian cancer individuals. Breast Cancer Res 2011;13(1):R20. [ Links ]
5. Szabo CI, King MC. Inherited breast and ovarian cancer. Hum Mol Genet 1995;4 (Spec No):1811-1817. [ Links ]
6. Broeks A, Urbanus JH, Floore AN, Dahler EC, Klijn JGM, Rutgers EJ, et al. ATM-heterozygous germline mutations contribute to breast cancer-susceptibility. Am J Hum Genet 2000;66:494-500. [ Links ]
7. Ahmed M, Rahman N. ATM and breast cancer susceptibility. Oncogene 2006;25:5906-5911. [ Links ]
8. Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 2003;3:155-168. [ Links ]
9. Lavin MF, Shiloh Y. The genetic defect in ataxia-telangiectasia. Annu Rev Immunol 1997;15:177-202. [ Links ]
10. González-Hormazábal P, Blanco R, Valenzuela C, Gómez F, Waugh E, Peralta O, et al. Association of common ATM variants with familial breast cancer in a South American population. BMC Cancer 2008;8:117. [ Links ]
11. FitzGerald MG, Bean JM, Hegde SR, Unsal H, MacDonald DJ, Finkelstein DM, et al. Heterozygous ATM mutations do not contribute to early onset of breast cancer. Nat Genet 1997;15:307-310. [ Links ]
12. Chen J, Birkholtz GG , Lindblom P, Rubio C, Lindblom A. The role of ataxia-telangiectasia heterozygotes in familial breast cancer. Cancer Res l998;58:1376-1379. [ Links ]
13. Milne RL. Variants in the ATM gene and breast cancer susceptibility. Genome Medicine 2009;1:12. [ Links ]
14. Mehdipour P, Mahdavi M, Mohammadi-Asl J, Atri M. Importance of ATM gene as a susceptible trait: predisposition role of D1853N polymorphism in breast cancer. Med Oncol 2011;28(3):733-737. [ Links ]
15. Gao L, Sun H, Wang X, Rao L, Li LJ, Liang WB, et al. The association between ATM Dl853N polymorphism and breast cancer susceptibility: a meta-analysis. J Exp Clin Cancer Res 2010;29:117. [ Links ]
16. Tapia T, Vallejos M, Alvarez C, Moraga M, Smalley S, Camus M, et al. ATM allelic variants associated to hereditary breast cancer in 94 Chilean women: susceptibility or ethnic influences? Breast Cancer Res Treat 2008;107(2):281-288. [ Links ]
17. Schrauder M, Frank S, Strissel PL, Lux MP, Bani MR, Rauh C, et al. Single nucleotide polymorphism DI853N of the ATM gene may alter the risk for breast cancer. J Cancer Res Clin Oncol 2008;134:873-882. [ Links ]
18. Angéle S, Moullan N, Vuillaume M, Chapot B, Friesen M, Jongmans W, et al. ATM haplotypes and cellular response to DNA damage: Association with breast cancer risk and clinical radiosensitivity. Cancer Res 2003;63:8717-8725. [ Links ]
19. Heikkinen K, Rapakko K, Karppinen SM, Erkko H, Nieminen, P,Winqvist R. Association of common ATM polymorphism with bilateral breast cancer. Int J Cancer 2005;116:69-72. [ Links ]
20. Tommiska J, Jansen L, Kilpivaara O, Edvardsen H, Kristensen V, Tamminen A, et al. ATM variants and cancer risk in breast cancer patients from Southern Finland. BMC Cancer 2006;6:209. [ Links ]
21. National Cancer Institute at National Institute of Health. Breast Cancer Risk Assessment Tool, [Accessed June, 2010]. Available at: http://www.cancer.gov/bcrisktool/ [ Links ]
22. Dork T, Bendix R, Bremer M, Rades D, Klopper K, Nicke M, et al. Spectrum of ATM gene mutations in a hospital-based series of unselected breast cancer patients. Cancer Res 2001;61:7608-7615. [ Links ]
23. Bretsky P, Gilad S, Yahalom J, Grossman A, Paglin S, Van Den Berg D, et al. The relationship between twenty missense ATM variants and breast cancer risk: The multiethnic cohort. Cancer Epidemiol Biomarkers Prev 2003;12:733-738. [ Links ]
24. Barrai I, Rodríguez-Larralde A, Dipierri J, Alfaro E, Acevedo N, Mamolini E, et al. Surnames in Chile: a study of the population of Chile through isonymy. Am J Phys Anthropol 2012;147(3):380-388. [ Links ]
25. Martínez-Cortés G, Salazar-Flores J, Fernández-Rodríguez LG, Rubi-Castellanos R, Rodríguez-Loya C, Velarde-Félix JS, et al. Admixture and population structure in Mexican-Mestizos based on paternal lineages. J Hum Genet 2012;57(9):568-574. [ Links ]
Received on: August 12, 2013
Accepted on: January 9, 2014
 Corresponding author:
Corresponding author:
Dr. Pablo Ruiz Flores.
Centro de Investigación Biomédica, Universidad Autónoma de Coahuila.
Gregorio A. García l98 sur. 27000 Torreón, Coahuila, México.
E-mail: pabloruiz@uadec.edu.mx
Declaration of conflict of interests. The authors declare that they have no conflict of interests.













