Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Salud Pública de México
Print version ISSN 0036-3634
Salud pública Méx vol.56 n.2 Cuernavaca Mar./Apr. 2014
Artículo original
Clinical and epidemiological features of extrapulmonary tuberculosis in a high incidence region
Características clínicas y epidemiológicas de la tuberculosis extrapulmonar en una región de alta incidencia
Carlos Pérez-Guzmán, MSc,(1) Mario H Vargas, MSc,(2,3) María del Rosario Arellano-Macías, RN,(1) Silvia Hernández-Cobos, RN,(1) Aurea Zelindabeth García-Ituarte, MD,(1) Francisco Javier Serna-Vela, MD.(1)
(1) Secretaría de Salud del Estado de Aguascalientes. Aguascalientes, México.
(2) Instituto Nacional de Enfermedades Respiratorias. México DF, México.
(3) Unidad de Investigación Médica en Enfermedades Respiratorias, Hospital de Pediatría, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social. México DF, México.
Abstract
Objective. To describe the clinical features of extrapulmonary tuberculosis (EXPTB) and to evaluate epidemiological data to search for potential explanations for its high frequency in the state of Aguascalientes, Mexico.
Materials and methods. Clinical records of all patients with tuberculosis seen in Aguascalientes in 2008 were reviewed, and official databases were analyzed.
Results. EXPTB comprised 60.5% of the 86 cases evaluated, being lymph nodes the main site affected. Patients with EXPTB were younger and more obese than subjects with pulmonary tuberculosis (PTB). One third of cases in either group had diabetes, a frequency much higher than expected. Epidemiological analysis showed that PTB incidence, but not EXPTB incidence, decreases as geographical altitude increases, and had a descendent trend from 1997 to 2011.
Conclusions. The lower frequency of PTB (due to its inverse relationship with altitude and its descendent trend in last years) might explain the high frequency of EXPTB. Obesity appeared to protect against developing pulmonary involvement, and diabetes was more frequent than expected among PTB and EXPTB cases.
Key words: Mycobacterium; epidemiology; demography; obesity; diabetes mellitus; Mexico.
Resumen
Objetivo. Describir las características clínicas de la tuberculosis extrapulmonar (TBEXP) y evaluar datos epidemiológicos para buscar posibles explicaciones de su alta frecuencia en Aguascalientes, México.
Material y métodos. Se revisaron expedientes de todos los pacientes con tuberculosis atendidos en Aguascalientes en 2008 y se analizaron bases de datos oficiales.
Resultados. La TBEXP constituyó 60.5% de los 86 casos evaluados, con afectación más común en ganglios linfáticos. Los pacientes con TBEXP fueron más jóvenes y más obesos que aquéllos con tuberculosis pulmonar (TBP). Un tercio de cada grupo tenía diabetes, una frecuencia muy superior a la esperada. El análisis epidemiológico mostró que la incidencia de TBP, pero no de TBEXP, es menor conforme aumenta la altitud geográfica y además está disminuyendo (l997-2011).
Conclusiones. La menor incidencia de TBP (por su relación inversa con la altitud y por su tendencia a disminuir en los últimos años) podría explicar la alta frecuencia de TBEXP. La obesidad parece proteger contra la afectación pulmonar, y la diabetes fue más frecuente de lo esperado tanto en TBP como en TBEXP.
Palabras clave: Mycobacterium; epidemiología; demografía; obesidad; diabetes mellitus; México.
In the Mexican state of Aguascalientes, the average incidence (x 100 000 inhabitants) of pulmonary tuberculosis (PTB) during the 2005-2009 period was 4.28, a figure well below the national average of 13.77 for the same period.1 In contrast, the mean incidence of extrapulmonary tuberculosis (EXPTB) was 4.38, which was much higher than the national average of 2.69. Thus, among the 32 Mexican states, Aguascalientes had the highest EXPTB/PTB ratio, in as much as EXPTB accounted for 50.6% of all cases of tuberculosis, compared with a national ratio of 16.3%. This exceedingly high frequency of EXPTB is also in contrast with the nearly 15% reported in different series worldwide.2
While extensive information has been gathered about factors predisposing to PTB, those related with the occurrence of EXPTB in individuals and populations have been less extensively investigated. Some studies have postulated that local EXPTB cases may be related with recent immigration3,4 or with mycobacteria other than Mycobacterium tuberculosis.5,6
Thus, due to the irregular behavior of tuberculosis in Aguascalientes, the aim of the present work was to describe the main demographic and health-related characteristics of patients with EXPTB, in comparison with those of patients with PTB, and to review national or regional epidemiological data in order to raise some potential explanations.
Materials and methods
This was a retrospective, descriptive, and cross-sectional study. Clinical records from all patients catalogued as a case of tuberculosis (pulmonary or extrapulmonary) by our national tuberculosis control program (NTP) and seen in the state of Aguascalientes, Mexico, during 2008 were evaluated. Data was obtained directly from the clinical record at the treating medical unit and by interviewing patients or their relatives if necessary. In Mexico, the diagnosis and treatment of any case of tuberculosis in any medical unit are conducted in accordance with World Health Organization recommendations, which are implemented nationwide by the NTP.7 Tuberculosis was diagnosed when subjects with clinical and/or imaging data compatible with tuberculosis had at least one of the following criteria: positive sputum smear for acid-fast bacilli (AFB); positive culture for M. tuberculosis; biopsy suggestive of tuberculosis, and/or full response to antitubercular treatment. These case definitions are in line with international guidelines. Cases with incomplete records or patients whose clinical record could not be located were excluded. The study protocol was approved by the Scientific and Ethics Committee of the Aguascalientes State Health Institute (approval 2HGTM-14/10).
Additionally, data on the nationwide epidemiology of pulmonary and extrapulmonary tuberculosis was obtained from the internet site of the Dirección General de Epidemiología,1 while altitude above sea level (weighted mean of major cities) was obtained from the Instituto Nacional de Estadística y Geografía.8 Information on other issues such as the prevalence of bovine tuberculosis, and the socioeconomic or health status of population from Aguascalientes or nearby regions was obtained from institutional or government publications.8-10
Data analysis
Chi square with Yates correction and Fisher exact tests were used to compare frequency variables between the two groups. Interval variables were evaluated according to their distribution by Student's t or Mann-Whitney U tests for pairwise differences, or by ANOVA and Tukey tests or Kruskal-Wallis and Dunn tests for multiple comparisons. The Pearson's correlation coefficient was also utilized. Statistical significance was established at p<0.05 bimarginally. Data was analyzed by using Epi-Info v6.0 software (CDC & WHO, Geneva, Switzerland).
Results
Clinical data
In the study period, 93 patients with tuberculosis seen in any medical unit of the Aguascalientes State Health System were identified. From this population, seven patients were excluded because their clinical records could not be located (4 cases) or these records had incomplete information (3 cases). Thus, our final sample comprised 86 patients, 52 (60.5%) with EXPTB forms (4 patients in this group also had positive AFB in sputum), and 34 (39.5%) with PTB, yielding an EXPTB/PTB ratio of 1.5:1.
In patients with EXPTB, the most commonly affected site was lymph nodes (42.3%), followed by abdominal cavity (including cases with diagnosis of abdominal, peritoneal or intestinal tuberculosis, 15.4%), skin (11.5%), pleura (7.7%), kidney and genitourinary system (7.7%), meninges (5.8%), disseminated (i.e., miliary tuberculosis, 5.8%) and joints (3.8%). In only two patients the diagnosis of EXPTB was made solely on clinical grounds, whereas in the remaining it was supported by histological (47 subjects), culture (7 subjects) or AFB in sputum (4 subjects) evidences (table I).
Table I depicts the main findings concerning personal, addictions, occupation, and tuberculosis-related data. Although a slight difference in the proportion of male and female patients was observed in the two forms of tuberculosis, this difference did not reach statistical significance (p=0.45). In relation to age, patients who developed extrapulmonary forms of TB were younger than those with pulmonary disease (31.6 vs. 53.5 years old, respectively, p<0.001). However, as can be noted in Figure 1A, age also varied among patients with extrapulmonary forms, with pleural and abdominal tuberculosis being relatively older than subjects with other EXPTB types. When pleural and abdominal forms were jointly analyzed, they did not differ in age from patients with TBP (Figure 1B). History of smoking or drug addiction was found in 21% and 12% of patients with PTB, respectively, while none of the patients with EXPTB had these habits (p=0.001 and p=0.02, respectively). The PPD skin test was positive in only 38.8% of patients with EXPTB and in up to 75% of subjects with PTB (p=0.003). Assessment of symptom duration before diagnosis unveiled a mild diagnostic delay in the PTB group (median, 4.5 months) in comparison with the EXPTB group (3.0 months) but this difference, although clinically important, was devoid of statistical significance. Figure 2 splits the time elapsed before diagnosis according to sites affected, with no statistical difference among them (p=0.13). All patients with PTB had positive AFB in sputum, and M. tuberculosis was cultured in sputum samples from three of them. In EXPTB cases, AFB in sputum was positive in four patients, and the sputum or biopsy culture was positive in seven patients (in one patient both tests were positive). Thus, microbiological confirmation of EXPTB was obtained in 10/52=19.2% patients. No mycobacteria other than M. tuberculosis were recovered from cultures.
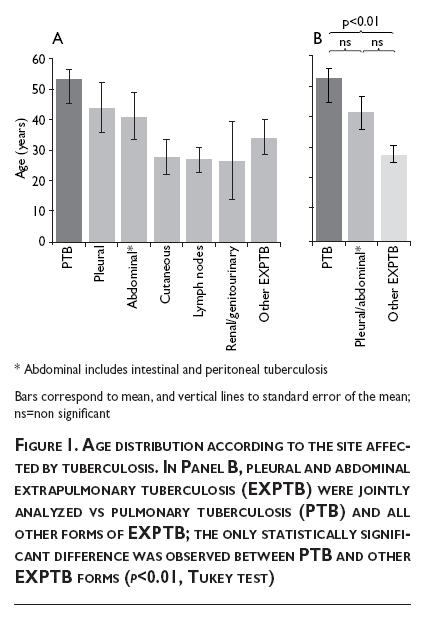
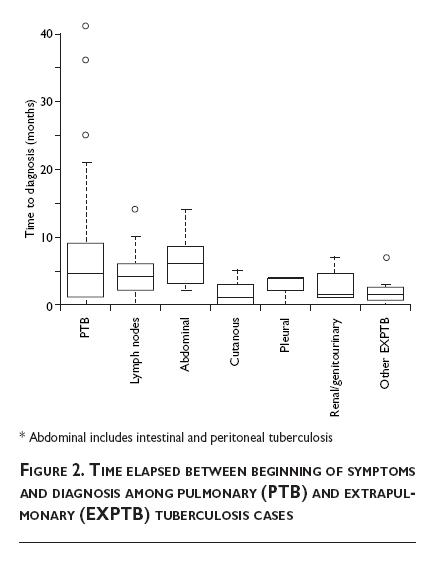
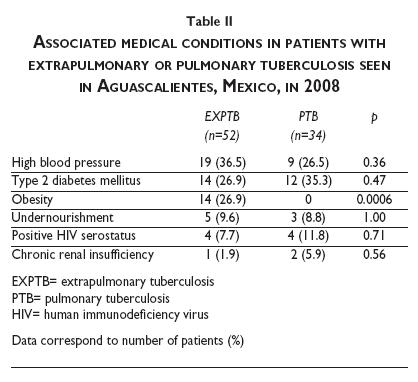
Table II illustrates the major co-morbidities among the study population. High systemic blood pressure and type 2 diabetes mellitus were the most frequent conditions, but these did not differ between both forms of tuberculosis. Regarding diabetes, although the PTB:EXPTB ratio was relatively higher among persons with diabetes (12:14, ratio of 0.86) than in patients without diabetes (22:38, ratio of 0.58), this variation was devoid of statistical significance. In contrast, while up to 26.9% of subjects with EXPTB had obesity (body mass index [BMI] ≥ 30 kg/m2), no patient with PTB was obese (p<0.001). The frequency of other conditions such as undernourishment, HIV serostatus, and chronic renal insufficiency were not different between both groups.
Epidemiological data
To evaluate the influence of immigration on the frequency of EXPTB in each of the 32 Mexican states (including the Distrito Federal), a correlation analysis was performed between recent immigration (percentage of people who moved to that state in the last five years) and the percentage of EXPTB cases in relation to all forms of tuberculosis (cumulative cases from 2005 to 2009). This analysis showed that the degree of recent immigration was unrelated with the frequency of EXPTB (r=0.05, p=0.78, not illustrated).
Because some years ago we reported that the incidence of PTB among the 32 Mexican states was inversely correlated with altitude above sea level,11 we now evaluated whether this association was also true for EXPTB. As illustrated in Figure 3, we corroborated that altitude had a strong negative correlation (r= -0.76, p<10-6) with PTB incidence (average 2005-2009), but not with the corresponding EXPTB incidence (r= -0.15, 0=0.41).
Finally, temporal trends (1997 to 2011) of EXPTB and PTB in Aguascalientes were compared with those of Guerrero, a Mexican state with one of the highest incidence of PTB, as well as with the nationwide trends (Figure 4). We found that in the three settings, EXPTB incidence was relatively stable (~3 x100 000 inhabitants). By contrast, PTB had a descendent trend in Aguascalientes (r=-0.86, p<0.001) and nationwide (r=-0.81, p<0.001), but not in Guerrero (r=-0.26, p=0.36).
Discussion
The incidence of EXPTB is usually considered lower than that of pulmonary cases. In the 2011 Report on Global Tuberculosis Control, the World Health Organization estimated that EXPTB accounted for 14.8% of all new cases of tuberculosis worldwide.2 Separated by regions, this percentage ranged from 4.6% in the Western Pacific Region to 21.8% in the Eastern Mediterranean Region. Among the 22 countries with the highest tuberculosis-burden, China had the lowest (0.7%) and Cambodia the highest (55.3%) proportion of EXPTB, respectively. Some large epidemiological studies are in agreement with these figures. For example, in USA, Peto et al. analyzed 253 299 registered cases of tuberculosis from 1993 to 2006, and concluded that only 18.7% were extrapulmonary forms.12 Likewise, te Beek et al. studied 13 258 patients with tuberculosis in The Netherlands from 1993 to 2001 and they found that 38% were EXPTB cases.4 Two recent reports from the European Union found that among the analyzed countries the proportion of EXPTB varied from 4-6 to 44-48% of all tuberculosis cases, and authors postulated that such variation might be due to differences in risk factors for EXPTB, challenges for diagnosing EXPTB,13 or decreased incidence of PTB.14
In our study, we found a high frequency of EXPTB in the Mexican state of Aguascalientes (60.5%), which is in line with official statistics and previous studies,1,15 but the reason for this behavior is unclear. Te Beek et al. in The Netherlands4 and Kruijshaar and Abubakar in Wales3 suggested that the increasing proportion of EXPTB observed in their countries was related with cases occurring in people born outside The Netherlands and Wales, respectively. However, immigration appears not to be the explanation in Mexico because, according to official statistics, the level of recent immigration in a particular state is not related with the percentage of EXPTB in that region. A second possibility is that mycobacteria other than M. tuberculosis were responsible for the EXPTB cases, for example, M. bovis transmitted from dairy cattle. Although the herd-raising activity is important in Aguascalientes, tuberculosis in cattle is currently under control (prevalence of bovine tuberculosis <0.10%).9 In addition, even though the number of cultures in our series was small (only 10 in the 52 EXPTB cases, i.e., 19%) M. tuberculosis was the only mycobacteria recovered in the seven positive cultures. This is in agreement with a recent study by Rodríguez-Núñez et al, in which 41 samples of tuberculous lymph nodes from patients residing in Aguascalientes were assessed by in situ hybridization.16 According to these authors, only M. tuberculosis was detected in all samples (author's personal communication). Contrasting with this study, Alvarado-Esquivel et al. performed PCR and molecular analysis for mycobacterial identification in biological samples from 72 patients with EXPTB in three states near Aguascalientes.17 Mycobacteria were confirmed in about one third of the samples, but a large percentage (~70%) of non-tuberculous species was identified. Thus, at present the possibility that mycobacteria other than M. tuberculosis were responsible for the EXPTB cases remains open.
A third possibility is related with geographical factors. In a previous study, we found that the incidence of PTB among the 32 Mexican states inversely correlated with altitude above sea level.11 We have now confirmed this relationship by using recent official tuberculosis notification statistics. However, EXPTB incidence rates were not affected by altitude. Thus, the contrasting behavior of PTB and EXPTB in relation to altitude might constitute a major factor in determining the proportion of EXPTB cases in a particular geographical region. In the case of Aguascalientes State, its main cities have a weighted mean altitude of 1 867 m above sea level; thus, its relatively low incidence of PTB might explain the nearly equal EXPTB:PTB ratio.
Finally, temporal trends clearly showed that the nationwide EXPTB incidence has been relatively unchanged in the last 15 years (1997 to 2011). Among the 32 Mexican states, in this time period 21 had an even trend, and only a few showed a downward (seven states) or upward (four states) trend (data not illustrated). By contrast, national PTB incidence is slowly descending as a consequence of the downward trends seen in many states, including Aguascalientes. Although these lower rates are surely due to the successful application of the national tuberculosis control program, paradoxically a failure to detect new PTB cases or their death prior to diagnosis might partially account for these lower trends. This epidemiological behavior is in agreement with a large study made by Sandgren et al.14 in 167 652 cases of EXPTB from the European Union. Like us, they found that the absolute number of notified EXPTB cases remained stable in the period 2002-2011, but notification of PTB cases decreased, leading to an increase in the proportion of EXPTB cases. A similar conclusion was obtained by García-Rodríguez et al.18 in the Spanish region of Galicia, where PTB cases showed a greater reduction rate than EXPTB cases during the study period (1991-2008), with a resulting increasing proportion of extrapulmonary cases.
Therefore, it seems that a modification of the PTB incidence (either due to geographical factors or as a consequence of temporal trends) is the main factor determining the percentage of EXPTB cases in a particular region.
Regarding gender, we found a mild predominance of male subjects among patients with EXPTB. Although this finding agrees with some studies,19 others have reported a small female predominance.20,21 On the other hand, subjects with EXPTB as a group were younger than those with pulmonary forms, which is in line with other published studies,20,22 although further analysis in our series showed that this was only true for EXPTB forms other than pleural and abdominal (figure 1). Some possibilities have been already outlined to explain the differences in age of subjects affected by PTB and EXPTB. For example, a relatively lesser maturation of the immune system might predispose younger subjects to develop EXPTB,23 or decreased lung immunity due to lifestyle factors (smoking) or co-morbid conditions (e.g., COPD) might predispose older subjects to PTB.22,24 However, as occurs with many other areas concerning knowledge of tuberculosis, the precise mechanisms remain elusive.
Three recent meta-analyses coincided that tobacco smoking is a risk factor for the development of PTB,25-27 but the association between tobacco and extrapulmonary forms of tuberculosis is still unclear. In our study, the smoking habit was absent among patients with EXPTB, contrasting with a 21% prevalence among subjects with PTB. This contrasting frequency of smoking has also been found by Sreeramareddy et al., who found an almost double frequency of ever smokers among patients with PTB, as compared with subjects with EXPTB (51.7 vs. 23.5%, respectively).22
In our study, the site most commonly affected by EXPTB was lymph nodes (42%), similar to that described in many other reports.12,16,19,20,22,28,29 The second most affected site, however, varies in different series. For instance, we found intestinal/abdominal/peritoneal tuberculosis in 15% of patients, whereas Peto et al.12 and Kourbatova et al.19 reported pleural disease (19.8 and 21%, respectively), and Nissapatorn et al.20 found disseminated tuberculosis. Although the reasons for these differences could have biological bases, other factors such as diagnostic capabilities or sociodemographic factors might also be responsible to some extent.
Symptom duration prior to diagnosis was shorter in patients with EXPTB than in PTB cases. There is scarce information on this issue currently available in the literature. Levine et al. described that symptoms of pleural tuberculosis were present for < 1 month in 62% of cases, whereas in our study, only 26% of subjects with EXPTB had symptoms for < 1 month.30 Factors responsible for this slight divergence in symptom duration might be related with differences in access to medical services as well as with sociocultural aspects.
We found that up to 26.9% of subjects with EXPTB were obese. This percentage closely matches the prevalence of obesity in Aguascalientes State among adults aged 20 years or over (26.7%),10 which suggests that EXPTB is not related with nutritional status. In contrast, a strong negative relationship between obesity and PTB was observed, inasmuch as no patient in this group was obese. At first glance, this might be explained by the conventional wisdom that PTB, as a chronic illness, leads to weight loss and malnutrition (and hence the ancient term phthisis, "wasting away"). However, the reverse relationship is also likely, i.e., that nutrimental deficiency favors the development of PTB. In this regard, Leung et al. studied a large cohort of 42 116 individuals aged 65 years or over with up to 5 years of follow-up, and these authors found that obesity was a protective factor against active PTB (odds ratio [OR]=0.30, 95% confidence interval [95%CI]: 0.11-0.84), but not for EXPTB (OR=1.20, 95%CI: 0.25-5.80).31 In line with this concept, biochemical variables often associated with obesity, such as hypercholesterolemia, have also been suggested as protective factors against development of PTB.32,33
In our study, diabetes and systemic hypertension were usual co-morbidities, but their respective frequencies did not differ in both forms of tuberculosis. However, while the 26.5% frequency of high blood pressure among patients with PTB was in agreement with the expected prevalence of this condition in Aguascalientes (27.5% in people aged 40 to 59 years),10 the 36.5% among subjects with EXPTB was much higher than expected (9.1% in persons 20-39 years of age).10 Rather than a risk factor per se, perhaps this excess proportion of hypertension among persons with EXPTB might be explained as due to the well-known association between high blood pressure and obesity.34 The frequency of diabetes was also much higher than expected, but this took place in both groups with tuberculosis (35.3% in PTB vs an expected 14.6% in persons aged 40-59 years, and 26.9% vs. an expected 2.5% in individuals aged 20-39 years).10 Diabetes is currently considered a predisposing factor for developing PTB,35,36 however, up to our knowledge this predisposition has not been reported for EXPTB, as our study suggests. Thus, additional research on this issue is warranted.
Conclusions
In Aguascalientes, EXPTB comprised 60.5% of all cases of tuberculosis. The reason for this high proportion of EXPTB is unclear, but might be related with the decreasing frequency of PTB as altitude above sea level increase, along with the descending trend of PTB incidence observed in the last years. Other factors, such as immigration or undernourishment, appear not to be involved
Clinically, patients with EXPTB were younger, with fewer addictions to tobacco or drugs and less positivity to PPD skin test, as compared with subjects with PTB. Obesity was absent in the PTB group, suggesting that this condition might protect subjects from developing pulmonary involvement. About one third of patients in either group had diabetes, a frequency that resulted much higher than expected in Aguascalientes. Our results pointed out that, along with the reinforcement of tuberculosis control programs, physicians should take into account the aforementioned clinical clues in order to improve their clinical ability to suspect EXPTB.
References
1. Centro Nacional de Vigilancia Epidemiológica y Control de Enfermedades. Anuarios de morbilidad. Mexico: Centro Nacional de Vigilancia Epidemiológica y Control de Enfermedades, 1984-2011. [ Links ]
2. World Health Organization. Global tuberculosis control: WHO report 2011. Geneva, Switzerland: WHO, 2011. [ Links ]
3. Kruijshaar ME, Abubakar I. Increase in extrapulmonary tuberculosis in England and Wales 1999-2006. Thorax 2009;64:1090-1095. [ Links ]
4. Te Beek LA, van der Werf MJ, Richter C, Borgdorff MW. [Increase in extrapulmonary tuberculosis in The Netherlands associated with an increase in the number of residents with non-Dutch nationality; observational study of data from 1993-2001]. Ned Tijdschr Geneeskd 2008;152:637-642. [ Links ]
5. Dankner WM, Davis CE. Mycobacterium bovis as a significant cause of tuberculosis in children residing along the United States-Mexico border in the Baja California region. Pediatrics 2000;I05:E79. [ Links ]
6. Lari N, Rindi L, Cristofani R, Rastogi N, Tortoli E, Garzelli C. Association of Mycobacterium tuberculosis complex isolates of BOVIS and Central Asian (CAS) genotypic lineages with extrapulmonary disease. Clin Microbiol Infect 2009;15:538-543. [ Links ]
7. World Health Organization. Treatment of tuberculosis: guidelines. 4th ed. Geneve: WHO, 2010. [ Links ]
8. Instituto Nacional de Estadística y Geografía. Censo de Población y Vivienda 2010: Principales resultados por localidad (ITER). México: INEGI, 2010. [ Links ]
9. Anonymous. Baja prevalencia de tuberculosis bovina. InfoRural; 2009. [ Links ]
10. Instituto Nacional de Salud Pública. Encuesta Nacional de Salud y Nutrición 2006. Resultados por entidad federativa, Aguascalientes. Cuernavaca, México. INSP, 2007. [ Links ]
11. Vargas MH, Furuya ME, Pérez-Guzmán C. Effect of altitude on the frequency of pulmonary tuberculosis. Int J Tuberc Lung Dis 2004;8:1321-1324. [ Links ]
12. Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis 2009;49:1350-1357. [ Links ]
13. Solovic I, Jonsson J, Korzeniewska-Kosela M, Chiotan DI, Pace-Asciak A, Slump E, et al. Challenges in diagnosing extrapulmonary tuberculosis in the European Union, 2011. Euro Surveill 2013;18(12):pii=20432. [ Links ]
14. Sandgren A, Hollo V, van der Werf MJ. Extrapulmonary tuberculosis in the European Union and European Economic Area, 2002 to 2011. Euro Surveill 2013;18(12). pii: 20431I. [ Links ]
15. De la Cruz J, Avelar FJ, Márquez F, Guerrero-Barrera AL. Panorama epidemiológico de la tuberculosis en Aguascalientes. Investigación y Ciencia 2008;40:38-41. [ Links ]
16. Rodríguez-Nuñez J, Avelar FJ, Márquez F, Rivas-Santiago B, Quiñones C, Guerrero-Barrera AL. Mycobacterium tuberculosis complex detected by modified fluorescent in situ hybridization in lymph nodes of clinical samples. J Infect Dev Ctries 2012;6:58-66. [ Links ]
17. Alvarado-Esquivel C, García-Corral N, Carrero-Domínguez D, Enciso-Moreno JA, Gurrola-Morales T, Portillo-Gómez L, et al. Molecular analysis of Mycobacterium isolates from extrapulmonary specimens obtained from patients in Mexico. BMC Clin Pathol 2009;9:1. [ Links ]
18. Garcia-Rodriguez JF, Alvarez-Diaz H, Lorenzo-Garcia MV, Marino-Callejo A, Fernandez-Rial A, Sesma-Sanchez P. Extrapulmonary tuberculosis: epidemiology and risk factors. Enferm Infecc Microbiol Clin 2011;29:502-509. [ Links ]
19. Kourbatova EV, Leonard MK, Jr., Romero J, Kraft C, del Rio C, Blumberg HM. Risk factors for mortality among patients with extrapulmonary tuberculosis at an academic inner-city hospital in the US. Eur J Epidemiol 2006;21:715-721. [ Links ]
20. Nissapatorn V, Kuppusamy I, Rohela M, Anuar AK, Fong MY. Extrapulmonary tuberculosis in Peninsular Malaysia: retrospective study of 195 cases. Southeast Asian J Trop Med Public Health 2004;35 Suppl 2:39-45. [ Links ]
21. Yang Z, Kong Y, Wilson F, Foxman B, Fowler AH, Marrs CF, et al. Identification of risk factors for extrapulmonary tuberculosis. Clin Infect Dis 2004;38:199-205. [ Links ]
22. Sreeramareddy CT, Panduru KV, Verma SC, Joshi HS, Bates MN. Comparison of pulmonary and extrapulmonary tuberculosis in Nepal- a hospital-based retrospective study. BMC Infect Dis 2008;8:8. [ Links ]
23. Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL. Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Respir J 2008;31:99-105. [ Links ]
24. Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum 2006;55:19-26. [ Links ]
25. Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch Intern Med 2007;167:335-342. [ Links ]
26. Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med 2007;4:e20. [ Links ]
27. Slama K, Chiang CY, Enarson DA, Hassmiller K, Fanning A, Gupta P, et al. Tobacco and tuberculosis: a qualitative systematic review and meta-analysis. Int J Tuberc Lung Dis 2007;11:1049-1061. [ Links ]
28. Cagatay AA, Caliskan Y, Aksoz S, Gulec L, Kucukoglu S, Cagatay Y, et al. Extrapulmonary tuberculosis in immunocompetent adults. Scand J Infect Dis 2004;36:799-806. [ Links ]
29. Ebdrup L, Storgaard M, Jensen-Fangel S, Obel N. Ten years of extrapulmonary tuberculosis in a Danish university clinic. Scand J Infect Dis 2003;35:244-246. [ Links ]
30. Levine H, Szanto PB, Cugell DW. Tuberculous pleurisy. An acute illness. Arch Intern Med I968;122:329-332. [ Links ]
31. Leung CC, Lam TH, Chan WM, Yew WW, Ho KS, Leung G, et al. Lower risk of tuberculosis in obesity. Arch Intern Med 2007;167:1297-1304. [ Links ]
32. Deniz O, Gumus S, Yaman H, Ciftci F, Ors F, Cakir E, et al. Serum total cholesterol, HDL-C and LDL-C concentrations significantly correlate with the radiological extent of disease and the degree of smear positivity in patients with pulmonary tuberculosis. Clin Biochem 2007;40:162-166. [ Links ]
33. Pérez-Guzmán C, Vargas MH, Quiñonez F, Bazavilvazo N, Aguilar A. A cholesterol-rich diet accelerates bacteriologic sterilization in pulmonary tuberculosis. Chest 2005;127:643-651. [ Links ]
34. Aghamohammadzadeh R, Heagerty AM. Obesity-related hypertension: Epidemiology, pathophysiology, treatments, and the contribution of perivascular adipose tissue. Ann Med 2012;44 Suppl 1:S74-S84. [ Links ]
35. Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis 2009;9:737-746. [ Links ]
36. Kapur A, Harries AD. The double burden of diabetes and tuberculosis - Public health implications. Diabetes Res Clin Pract 20I3;pii: S0168-8227-(12)00497-4. [ Links ]
Received on: December 10, 2012
Accepted on: November 13, 2013
 Corresponding author:
Corresponding author:
Dr. Mario H. Vargas.
Instituto Nacional de Enfermedades Respiratorias.
Tlalpan 4502. 14080 México DF, México.
E-mail: mhvargasb@yahoo.com.mx
Declaration of conflict of interests. The authors declare that they have no conflict of interests.













