Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Salud Pública de México
versión impresa ISSN 0036-3634
Salud pública Méx vol.55 no.5 Cuernavaca sep./oct. 2013
Artículo original
Hemoglobin A1c: A reliable and accurate test for diabetes care? A prospective study in Mexico
Hemoglobina A1c: ¿una prueba precisa y confiable para el cuidado de la diabetes ? Estudio prospectivo en México
José Gerardo González-González, MD, PhD,(1) René Rodríguez-Gutiérrez, MD,(2), Fernando Javier Lavalle-González, MD,(1) Arnulfo González-Cantú, MD,(1) Héctor Eloy Taméz-Pérez, MD,(1), Gerardo González-Saldívar, MD,(2) Jesús Zacarías Villarreal-Pérez, MD.(1)
(1) Servicio de Endocrinología, Hospital Universitario Dr. José E. González, Universidad Autónoma de Nuevo León. Monterrey, Nuevo León, México.
(2) Servicio de Medicina Interna, Hospital Universitario Dr. José E. González, Universidad Autónoma de Nuevo León. Monterrey, Nuevo León, México.
Abstract
Objective. To compare the concordance correlation coefficient for HbA1c results in an in-field experience.
Materials and methods. A prospective study in Monterrey, Mexico from April to August 2012 was conducted to evaluate the day-to-day clinical situation when measuring HbA1c. Blood samples from 38 consecutive patients were sent to seven local laboratories and one international reference laboratory.
Results. Poor concordance was found in 4 out of 7 laboratories, moderate in 2 out of 7, and significant in just one. HbA1c values from three laboratories fluctuated more than 1% above or below the reference laboratory in more than 30% of cases, and more than 2% in 10%-20% of subjects.
Conclusions. Standardized HbA1c measurement has not occurred worldwide. Physicians should be aware of this issue and be cautious of HbA1c guidelines on diabetes diagnosis or management until proper standardization programs are implemented.
Key words: diabetes mellitus; hemoglobin A glycosylated; laboratory test; Mexico.
Resumen
Objetivo. Comparar el coeficiente de correlación de concordancia de HbA1c.
Material y métodos. Estudio prospectivo en Monterrey, México, de abril a agosto de 2012 para evaluar la medición de la HbA1c. Participaron 38 individuos y se envió la muestra a 7 laboratorios locales y a uno internacional de referencia.
Resultados. Se encontró pobre concordancia en 4 de 7 laboratorios, moderada en 2 y una concordancia significativa en uno. Los valores de HbA1c de tres laboratorios fluctuaron más de 1% del laboratorio de referencia en más de 30% de los casos y más de 2% en 10 a 20%.
Conclusiones. La estandarización de la HbA1c no está concluida. Los médicos deberían tomar con cautela las recomendaciones de las guías para HbA1c en el diagnóstico o manejo de la diabetes hasta que se implementen los programas de estandarización.
Palabras clave: diabetes mellitus; hemoglobina A glucosilada; prueba de laboratorio; México.
β-N-(1-deoxy)-fructosyl hemoglobin, better known as hemoglobin A1c (HbA1c), reveals the mean blood glucose value in the past 8 to 12 weeks.1,2 It is widely recognized as a keystone in the assessment of the quality of chronic glycemic control and a treatment goal in diabetes care, predicting the risk of severe hypoglycemia and being a constant primary endpoint in diabetes clinical trials.3-5 The results of the DCCT (Diabetes Control and Complications Trial) and UKPDS (United Kingdom Prospective Diabetes Study) showed that A1c correlated remarkably to predict chronic microvascular complications and demonstrated a trend to predict macrovascular disease.6,7 Recently, international diabetes organizations have also given support to its use for diabetes screening and diagnosis, although this issue remains debatable.8-10 Therefore, a reliable and accurate assay for HbA1c measurement, available worldwide, is required for its ideal use.
Recognition of the clinical value of HbA1c in diabetes management led to the creation, around 1990, of different working groups to develop a global reference method as well as a standardization of the HbA1c measurement.11-13 Some countries achieved a national direct comparison method improving the variation of their HbA1c results but the need for a global strategic program was recognized.14 Therefore, since 1993, multiple meetings have been carried out by working groups of the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), the National Glycohemoglobin Standardization Program (NGSP), the American Diabetes Association (ADA), the International Diabetes Federation (IDF) and the European Association for the Study of Diabetes (EASD).15-19 There have been many advances, such as a global HbA1c reference system, standardization of the procedure and an agreement to inform the results, but the application of these recommendations has not been carried out in some countries that have large populations and a high prevalence of diabetes, such as Mexico.20 Furthermore, despite the fact that all scientific communications mention the rigorous need of a standardized HbA1c measurement procedure, this has failed to take place in some countries. Many Mexican healthcare providers, on a daily basis, likely rely on a non-standardized HbA1c test for diabetes screening, diagnosis, and treatment.8,12,13
As a consequence, we decided to carry out a prospective study to evaluate the day-to-day clinical situation that a type 2 diabetes Mexican patient faces when a blood sample for HbA1c measurement is taken. The primary endpoint was to compare the intraindividual/interlaboratory concordance correlation coefficient (CCC) for HbA1c. Secondary end points were: 1) to estimate the HbA1c CCC and dispersion data at two HbA1c range values and between different laboratories that shared the same HbA1c measurement technique, and 2) to determine the clinical significance of the intraindividual HbA1c results by different laboratories in the physician’s interpretation of glycemic control.
Materials and methods
Subjects
We studied 38 consecutive participants from April to August of 2012 of the Diabetes Clinical Research Unit of the Dr. José E. González University Hospital in Monterrey, Mexico. Approval was obtained from the Institutional Review Board and informed consent was obtained from all participants. Male or female patients between 18 and 70 years of age with diabetes mellitus were included. Pregnant women and all clinically well-recognized situations that may lead to erroneous HbA1c values were excluded.1 After an overnight fasting, a blood sample was taken for HbA1c measurement. The sample was sent to eight laboratories.
Measurements
After an overnight fast, a blood sample for HbA1c was taken between 0800 and 0900 hours in all participants and then sent to each laboratory within the next three hours at a temperature between 4 and 8ºC. The seven laboratories were the largest routine clinical laboratories in the metropolitan area of Monterrey, Mexico. Table I shows the methods for HbA1c determination used in each selected clinical laboratory. Three used ionic exchange high-pressure liquid chromatography (IE-HPLC) with different commercial products; two laboratories used cationic exchange resin by spectrophotometry (S-CER); one laboratory used an immunoassay of cationic exchange resin (I-CER), and the last one utilized turbid metric immunoassay (TI). The manufacturer for IE-HPLC (D10 short and extended) was Bio-Rad Laboratories, clinical diagnostics group and for G8 Tosoh was Bioscience Inc. The manufacturer for S-CER was Stanbio laboratory. The manufacturer for I-CER (DCA 2000) was Siemens healthcare diagnostics Inc., and for TI, Biolabo. As a reference for comparison, the eighth laboratory was a central, certified laboratory for HbA1c measurement in the United States (Quintiles, Durham, NC). This laboratory used the IE-HPLC technique (Bio-Rad variant II turbo A1c, Bio-Rad laboratories clinical diagnostics group). The intraassay and interassay coefficient of variation (CV) of the laboratories participating in this study are shown in Table I.
Statistical analysis
All results are reported as means ± standard deviations unless otherwise indicated. A p ≤ 0.05 was considered statistically significant. Descriptive statistical analysis was used for quantitative variables, measures of central tendency and dispersion. In the case of qualitative variables, frequencies were obtained. For concordance correlation analysis, Lin CCC was calculated.21 In the case of continuous variables, such as in our study, a value greater than 0.99 means almost perfect concordance, between 0.95 and 0.99 significant concordance, between 0.90 and less than 0.95, moderate concordance and when less than 0.90, poor concordance.21 The Bland & Altman method was used to show agreement between each local clinical laboratory and the reference laboratory. Using a formula for means equivalence studies with a K value (z α +z β)2 of 13, an alpha value of 0.05 two-tailed, a power of 95%, accepting an error of 1, a sample size of 31 participants was calculated. The statistical analysis was performed with IBM SPSS Statistics 20.0 and MedCalc Software bvba.22
Results
Study population
The mean age of the participants was 45.7 ± 12.2 years (range, 19-66). Twenty-eight cases (73.7%) were women. All participants had type 2 diabetes. Selecting the result of the reference laboratory, there were nine participants with an HbA1c value between 5.0 and 7.0%. A value greater than 7.0% and below 9.0% was found in 19 cases, and a value greater than 9.0 % but less than 11.0% was found in 10 subjects. There were no cases with a value greater than 11.0%.
Variation, dispersion and concordance of the HbA1c results
Table II shows the CV and CCC in the HbA1c results of the local clinical laboratories when compared to the reference laboratory. Laboratory 7 had the highest CV (30.35%). On the other hand, the lowest CV was found in laboratory 4 (15.28%). Furthermore, CCC found a poor concordance in 4 out of 7 laboratories (laboratory 1 (IE-HPLC), four (S-CER), six (IE-HPLC) and seven (S-CER), (0.84, 0.59, 0.65 and 0.50, respectively); a moderate concordance in 2 out of 7 [three (IE-HPLC) and five (TI)], and a significant but not almost perfect concordance (CCC= 0.95) only in laboratory 2 (I-CER).
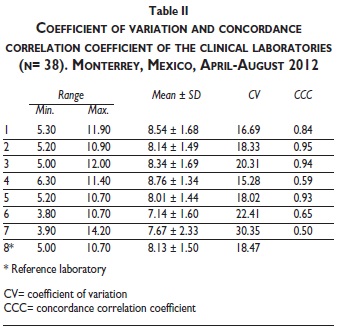
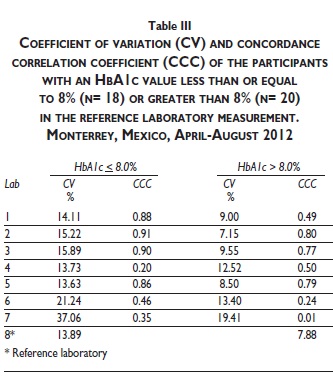
Table III shows the CV and CCC of the local laboratories when the participants were divided into two groups, selected by an HbA1c cutoff value of 8.0% in the reference laboratory. There was not a trend to better agreement of any local clinical laboratory in HbA1c values higher or lower than 8.0% in the reference laboratory. In values lower or equal than 8.0% in the reference laboratory, the CCC decreased in all three laboratories that had a better performance as a whole. The CCC of laboratory three remained in the same category (moderate concordance) but laboratory 2 and 5 showed a worse performance (two from significant to moderate concordance, and five from moderate to poor concordance). In HbA1c values higher than 8.0% the concordance with the reference laboratory was worse. No laboratory showed excellent, significant or moderate concordance. Laboratories that had a better performance as a whole (2, 3 and 5) all had poor concordance. The CCC of HbA1c values reported by the three local laboratories that used the IE-HPLC technique (laboratories 1, 3 and 5) was also analyzed. Laboratory 3 had a moderate concordance (CCC=0.91), and laboratory 1 and 6 had a poor concordance (CCC= 0.83 and 0.60, respectively).
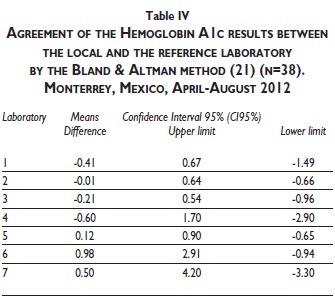
The agreement assessment between the local and the reference laboratory was also analyzed by the Bland & Altman method. Laboratories 2, 3 and 5 had the best agreement again (Table IV). As a whole, laboratory 2 showed the best agreement again with a means difference of −0.01 (95% confidence interval 0.64 to -0.66). Laboratories 4, 6 and 7 had unacceptable HbA1c agreement values.
Clinical impact of the disagreement in HbA1c results
Classification of the differences in the HbA1c results in each patient between the reference laboratory and each local laboratory is shown in Table V. The HbA1c results were classified into four categories of range dissimilarity: 1) equal or less than ± 0.5%, 2) ± 0.6 to 1.0%, 3) ± 1.1 to 2.0% and 4) greater than ± 2%. Laboratory 2 had the best plus/minus percent fluctuation equal or less than 0.5% in the HbA1c result (92%, 35 out of 38 cases). Laboratories 3 and 5 had 33 and 32 out of 38 cases, 87 and 84%, respectively. Laboratories 4, 6 and 7 had this category in less than a third of the cases (31, 29 and 29%, respectively). Laboratories 2 and 5 had any case with a plus/minus fluctuation in HbA1c greater than 1.0%. Laboratories 4, 6 and 7 had a plus/minus fluctuation in HbA1c greater than 1.0% in 32, 45 and 54% of the cases, respectively. A fluctuation greater than plus/minus 2.0% in HbA1c occurred in 11% (4 out of 38), 16% (6 out of 38) and 21% (8 out of 38) of the cases, respectively. Most cases in laboratory 4 had an HbA1c result above the result (greater than 1.0%) of the reference laboratory. The opposite was found in laboratory 6 and 7, which had an HbA1c result below the value (greater than 1.0%) of the reference laboratory.
Discussion
In our study, blood samples from 38 participants sent to seven of the most important local laboratories in a metropolitan area in a large city in Mexico and to one reference laboratory showed that 4 out of 7 laboratories had a poor concordance or agreement by different statistical methods and analytical procedures. This lack of agreement resulted in a greater lack of precision or reliability with higher HbA1c values when population was divided into cases above or below 8.0%. These findings reveal a huge problem in diabetes management in our large population, that could be present in many other communities or countries in daily clinical practice, since the characteristics of our study design is illustrative of a real-life type 2 diabetes patient when HbA1c is measured. Many clinical situations are well-recognized causes of misleading values of HbA1c.1 Variations in the lifespan of the erythrocyte and the molecular structure of hemoglobin, chronic renal failure, medications, ethnicity, aging, etc., are some of the most common etiologies. The physician should take all these situations into consideration in day-to-day clinical practice of type 1 and type 2 diabetes patients when assessing the quality of chronic glycemic control by an HbA1c measurement. Nevertheless, incorrect HbA1c values, in a wide error range, due to problems in laboratory measurements are not easy to suspect and could lead the physician to make a mistake in the classification of the quality of glycemic control and the decision-making process on pharmacological management in patients with diabetes.
For more than two decades, ADA, IDF, EASD, IFCC and representatives of different areas related to diabetes care have proposed global recommendations concerning HbA1c measurement methods and how the results should be informed.13-19 Three conclusions have been the core in almost all these technical reports: 1) "all HbA1c test results should be standardized worldwide, including the reference system and the method used for reporting the results", 2) "the new IFCC reference system for HbA1c represents the only valid tool for obtaining standardized HbA1c measurements," and 3) "HbA1c results should be reported everywhere in IFCC units (mmol/mol) and derived NGSP units (%), using the IFCC-NGSP master equation". A recent paper has mentioned that the first recommendation has been completed worldwide.23 Our results and other recent publications in different countries have recognized severe problems in this regard and their comments are in opposition to the statement of a completed globalization in HbA1c measurement.24-26 Again, our evaluation, based on an experimental model of daily clinical practice, in an adequate sample size, has shown a critical issue concerning diabetes management by different statistical methods; a very poor concordance or agreement in HbA1c results was found in more than half of the participating laboratories. The robust clinical significance of our results would not change with involvement of more clinical laboratories or subjects. Our study was carried out in the largest clinical laboratories of the metropolitan area of a big community in Mexico. Some studies have revealed concordance with some of our findings.27-30 A recent recommendation suggests that a 0.5% fluctuation range in HbA1c values is the acceptable limit for HbA1c measurement.13 Our results show that almost 2/3 of the cases in 4 out of 7 of our participating laboratories reported an HbA1c error value greater than 1.0% above or below the reference value. This finding makes HbA1c measurement unacceptable in our population in a daily clinical practice. A limitation of our study is the lack of participation of other cities in Mexico; it is necessary to extend this study to other countries. It is likely that our medical scenario is still occurring in many places in the world with high diabetes prevalence. The lack of this valuable assessment tool makes it very difficult to follow international diabetes screening diagnosis and management recommendations.8,9,31 It is clear that the recent recommendation of HbA1c measurement for diabetes screening and diagnosis is not conceivable to be applied in countries that share the same problematic uncovered by our study.8 Diabetes screening and diagnosis were not an endpoint in our study but our results in a type 2 diabetes population clearly show that HbA1c measurements cannot be used for all these purposes. As a consequence, it would be urgent that health authorities in many countries linked to these international academic organizations in diabetes carry out an international certification procedure of clinical laboratories to obtain a license for standardized HbA1c measurement.
The third statement is that HbA1c should be reported in IFCC units and derived NGSP units.13-19 IFCC units are not widely used by physicians on daily clinical practice and teaching institutions in our country. Patients are still less aware of the use of IFCC units. A technical report on HbA1c has mentioned that from January 2012, all scientific communications in prestigious journals were going to report HbA1c values in IFCC units and derived NGSP (%) units.13 We carried out a critical review of the 20 highest impact factor journals on diabetes and some prestigious internal medicine journals on articles published during this year (2012) and found that 90% are still using NGSP (%) units for reporting HbA1c values. Most physicians related with diabetes care are not aware of the advantages of this new way to report HbA1c results but this issue increases the complexity of the implementation of strategies for HbA1c clinical interpretation and standardization all over the world.
In conclusion, in some locations there is still a serious problem in HbA1c measurements as a result of a lack of standardization of the methods in clinical routine laboratories. This situation makes it difficult to apply many important recommendations on diabetes patient management and, as a consequence, patients are at greater risk of developing chronic diabetes complications. In the case of diabetes screening or diagnosis, the unreliability and dispersion of our HbA1c findings makes this strategy a risk for under or over diagnosis of diabetes. All recommendations or guidelines related to HbA1c use in clinical practice must be strong and clear, pointing out that they apply only with standardized laboratories. In daily clinical practice, however, physicians do not ask whether the HbA1c result comes from a standardized laboratory or not. It is necessary to carry out randomized studies to evaluate the reliability of the HbA1c results in everyday management of patients with diabetes, particularly in countries that are likely in the same situation as us. Once this situation has been solved, it could be valuable to go for implementation of HbA1c in IFCC units and other issues with a less valuable clinical relevance in day-to-day diabetes care.
Acknowledgments
We wish to thank Dr. Sergio Lozano-Rodríguez and Dr. José Juan Segura-Luna from the Research Department of the University Hospital Dr. José E. Gonzalez, for their critical reading of the manuscript and statistical advice, respectively.
References
1. Gough S, Manley S, Stratton I, eds. In:HbA1c in diabetes. Case studies using IFCC units. Introduction. Hoboken NJ: Blackwell Publishing Ltd, 2010:1-28. [ Links ]
2. Dailey G. Assessing glycemic control with self-monitoring of blood glucose and hemoglobin A1c measurements. Mayo Clin Proc 2007;82:229-236. [ Links ]
3. Woo V, Shestakova MV, Orskov C, Ceriello A. Targets and tactics:the relative importance of HbA1c, fasting and postprandial plasma glucose levels to glycaemic control in type 2 diabetes. Int J Clin Pract 2008;62:12:1935-1942. [ Links ]
4. Marshall SM. Standardization of HbA1c:good or bad? Nat Rev Endocrinol 2010;6:408-411. [ Links ]
5. Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia 2007;50:2239-2244. [ Links ]
6. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-986. [ Links ]
7. UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-853. [ Links ]
8. Nathan DM, Balkau B, Bonora E, Borch-Johnsen K, Buse JB, Colagiuri S, et al. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327-1334. [ Links ]
9. Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of type 2 diabetes:a systematic review Diabet Med 2007;24:333-343. [ Links ]
10. Kramer CK, Araneta MR, Barrett-Connor E. A1c and diabetes diagnosis:The rancho San Bernardo Study. Diabetes care 2010;33:101-103. [ Links ]
11. Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE. The National Glycohemoglobin Standardization Program:a five-year progress report. Clin Chem 2001;46:1985-1992. [ Links ]
12. Hoelzel W, Miedema K. Development of a reference system for the international standardization of HbA1c/glycohemoglobin determinations. Int Fed Clin Chem 1996;9:62-67. [ Links ]
13. GLAD working group. Recommendations for the implementation of international standardization of glycated hemoglobin in Italy. Clin Chem Lab Med 2010;48:623-626. [ Links ]
14. Berg AH, Sacks DB. Haemoglobin A1c analysis in the management of patients with diabetes:from chaos to harmony. J Clin Pathol 2008;61:983-987. [ Links ]
15. Consensus statement on the worldwide standardization of the hemoglobin A1c measurement:the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care 2007;30:2399-2400. [ Links ]
16. Mosca A, Goodall I, Hoshino T, Jeepson JO, John WG, Little RR et al. Global standardization of glycated hemoglobin measurement:the position of the IFCC Working Group. Clin Chem Lab Med 2007;45:1077-1080. [ Links ]
17. Manley SE. Estimated average glucose derived from HbA1c (eAG):report from European Association for the Study of Diabetes (EASD), Amsterdam 2007. Diabet Med 2008;25:126-128. [ Links ]
18. Leslie C. LAI. Global standardization of HbA1c. Malasyan J Pathol 2008;30:67-71. [ Links ]
19. Hanas R, John G. 2010 Consensus statement on the worldwide standardization of the hemoglobin A1c measurement. Diabetes Res Clin Pract 2010;90:228-230. [ Links ]
20. Villalpando-Hernández S, Cruz V, Rojas R, Shamah-Levy T, Ávila MA, Gaona B, et al. Prevalence and distribution of type 2 diabetes mellitus in Mexican adult population. A probabilistic survey. Salud Publica Mex 2010, 52:19-26. [ Links ]
21. Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989;45:255-268. [ Links ]
22. Schoonjans F, Zalata A, Depuydt CE, Comhaire FH. MedCalc:a new computer program for medical statistics. Comput Methods Programs Biomed 1995:48;257-262. [ Links ]
23. Inzucchi SE. Diagnosis of diabetes. N Engl J Med 2012;367:542-550. [ Links ]
24. Beer KA. A1c assay emerges as the new standard diagnostic test. JAAPA 2010;23:50-51. [ Links ]
25. Llanes de Torres R. Glicada para el diagnóstico de la diabetes, ¿un estándar universal? Aten Primaria 2010;42:571-576. [ Links ]
26. Álvarez-García E. HbA1c, estandarización y expresión de resultados. Endocrinol Nutr 2010;57:177-181. [ Links ]
27. Holmes EW, Ersahin C, Augustine GJ, Charnogursky GA, Gryzbac M, Murrell JV, et al. Analytical bias among certified methods for the measurement of hemoglobin A1c. A cause for concern? Am J Clin Pathol 2008;129:540-547. [ Links ]
28. Rojano-Rodríguez E, Acosta-González RI, Bocanegra-Alonso A, Rivera-Sanchez G, Sierra-Amor RI. Desempeño de un grupo de laboratorios mexicanos en la determinación de HBA1C. Bioquimia 2007;32:91-99. [ Links ]
29. Schweitzer M, Cavan DA, Ziegler R. Is HbA1c a reliable measure for assessing glycaemic control? Diabetologia 2012;55:Suppl 1:S1-S538. [ Links ]
30. Méndez-Chacón E, Rosero-Bixby L, Fernández-Rojas X, Barrantes-Jimenes K. Comparación de los resultados de pruebas de laboratorio seleccionadas de un estudio poblacional de adultos mayores de Costa Rica. Población y Salud en Mesoamérica 2007;5:2-15. [Accessed April 2012]. Disponible en: http://www.ccp.ucr.ac.cr/revista/. [ Links ]
31. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M. Management of hyperglycemia in type 2 diabetes:a patient-centered approach:position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35(6):1364-1379. Epub 2012 Apr 19. [ Links ]
Received on: December 5, 2012
Accepted on: June 14, 2013
 Corresponding author:
Corresponding author:
Dr. José Gerardo González-González.
Servicio de Endocrinología, Hospital Universitario Dr. José E. González,
Universidad Autónoma de Nuevo León. Av. Madero y Gonzalitos s/n,
col. Mitras Centro. 64460 Monterrey, NL, México.
E-mail: jgonzalezg@investigacion-meduanl.com
Declaration of conflict of interests. The authors declare that they have no conflict of interests.













