Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Salud Pública de México
versión impresa ISSN 0036-3634
Salud pública Méx vol.53 supl.1 Cuernavaca ene. 2011
ARTÍCULO DE REVISIÓN
Viral hepatitis infection and insulin resistance: a review of the pathophysiological mechanisms
Hepatitis virales y resistencia a la insulina: revisión de los mecanismos fisiopatológicos
Ylse Gutiérrez-Grobe, MDI; Guadalupe Ponciano-Rodríguez, MsCII; Nahum Méndez-Sánchez, MD, PhDI
IClínica Médica Sur. México DF, México
IIUniversidad Nacional Autónoma de México, Facultad de Medicina, Departamento de Investigación en Salud Pública. México DF, México
ABSTRACT
Viral hepatitis is a common cause of morbidity in Mexico. Insulin resistance (IR) is related to the liver damage caused by some viral infections, especially chronic infections. Chronic viral infection is an important risk factor for the development of type 2 diabetes mellitus, disease that is currently among the 10 main causes of morbidity and the most common cause of mortality. Although several studies have reported an association between IR and hepatitis B virus or hepatitis C virus (HCV) infection, the pathophysiology has been studied thoroughly only for the association between IR and HCV infection. It is thought that HCV infection causes direct damage through the action of the core proteins, which induces an inflammatory state characterized by secretion of proinflammatory cytokines that interfere with normal insulin signaling and disturb glucose, lipid and protein metabolism. This review summarizes the mechanisms by which viral infection is thought to induce IR.
Key words: insulin resistance; hepatitis B virus; hepatitis C virus.
RESUMEN
Las hepatitis virales son una causa común de morbilidad en México. La resistencia a la insulina (RI) ha sido relacionada con el daño hepático causado por infecciones virales crónicas, haciendo de ellas un factor de riesgo para el desarrollo de diabetes mellitus tipo 2, problema de salud que se encuentra entre las primeras 10 causas de morbilidad y es la primera de mortalidad. Aunque varios estudios han reportado una asociación entre la RI y la infección con virus de la hepatitis B y virus de la hepatitis C, sólo con el último se ha estudiado su fisiopatología. Se ha sugerido que produce daño directo a través de proteínas de su núcleo e induce un estado inflamatorio que interfiere con la señalización normal de insulina, resultando en una alteración del metabolismo de glucosa, lípidos y proteínas. Esta revisión resume los mecanismos por los que se sugiere que estas infecciones inducen RI.
Palabras clave: resistencia a la insulina; virus de hepatitis B; virus de hepatitis C.
Many agents are capable of causing liver damage, including drugs, toxins, infections, and metabolic alterations, any of which may lead to liver failure. Viral infection is a frequent cause of liver damage. Viral infections considered to be hepatotropic include viral hepatitis caused by hepatitis viruses A, B, C, D, E, and F,1 while the non-hepatotropic are Cytomegalovirus, Herpes Virus, and Epstein-Barr Virus. Most produce an acute infection, and only hepatitis viruses B, C, D, and E cause chronic liver disease. Several studies have shown that the damage or metabolic changes induced by these viruses have a significant impact on several organs of the body besides the liver. In recent years, insulin resistance (IR) and the metabolic syndrome have become more important and are linked to liver diseases, especially chronic diseases. The pathophysiological basis of the metabolic syndrome is IR, which is defined as an increased need for insulin in the peripheral tissues (muscle and adipose) to achieve normal blood glucose levels and to reduce the glucose output in the liver,2 and may lead to type 2 diabetes mellitus (T2DM). In this paper, we explain the pathophysiology of IR caused by infection with viral agents responsible for liver damage.
Epidemiology
Viral hepatitis infection is common in Mexico and its prevalence has been increasing. In 2008, 1 107 cases of infection with hepatitis B virus (HBV) and 2 226 cases of hepatitis C virus (HCV) were reported, giving incidence rates of 1.04 and 2.09 per 100 000 inhabitants, respectively.3 The number of cases is expected to continue increasing in the coming decades.4
Overweight and obesity are major public health issues and are considered risk factors for the development of IR and the metabolic syndrome, which are the physiological bases of T2DM, another pathological entity whose prevalence has been increasing. In 2008, 396 374 new cases were reported, giving an incidence rate of 371.4 per 100 000 inhabitants; most patients are older than 45 years.3
Recent studies have confirmed a close relationship between IR and other liver diseases. IR and T2DM are associated with complications similar to those associated with chronic hepatitis caused by HBV and HCV infection, such as cirrhosis and hepatocellular carcinoma. Zein and colleagues reported that T2DM occurs with a high prevalence in cirrhotic patients in both Western and Eastern countries. The prevalence of T2DM is especially high in cirrhotic patients whose etiology is HCV infection rather than alcohol or cholestatic disease, etiologies in which is also noted a greater incidence of T2DM than in the general population (figure 1).5 The prevalence rates of T2DM in HCV infection is high: up to 33% in the USA,6 23% in Spain,7 20% in Japan8 and 22% in Saudi Arabia9 have been reported. A cross-sectional study performed by our group in a university hospital in Mexico City found a similar prevalence of this association of 22.7%.10 The prevalence rates of T2DM in HBV infection is of 12% in the USA,11 11.9% in Japan,8 and 16.7% in Mexico.10,12,13
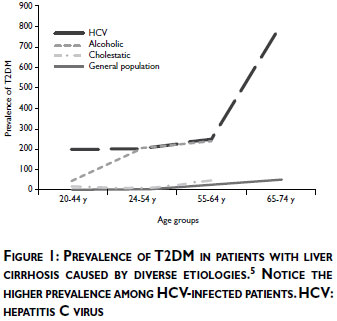
The relationship between chronic liver diseases and T2DM is strong enough to be called "hepatogenous diabetes", which is now recognized by the World Health Organization as an independent entity involving the development of T2DM caused by cirrhosis.14 It has been suggested that 17-30% of cirrhotic patients are clinically diabetic and that this entity is caused by two abnormalities that occur simultaneously: IR in muscle and the inadequate response of β-cells, which secrete insufficient insulin to overcome the defect in insulin action. The IR induced by liver cirrhosis is greater than that caused by T2DM and leads to faster onset of and more severe metabolic complications.15
Liver-related IR
Figure 2 shows the mechanisms of IR related to the liver. Two factors may explain IR of liver origin. One pathway is through the production of free fatty acids; an increase in their concentration inhibits the insulin-induced suppression of endogenous glucose production,16 and the stimulation of gluconeogenesis17 associated with activation of protein kinase Cδ.18
Another pathway associated with IR in the liver is mutation of fructose 2,6-bisphosphonatase, which leads to a decrease in fructose 2,6-bisphosphonate, which causes three changes: a less suppression of hepatic glucose production (increased gluconeogenesis), disruption of glucose flux, and a decrease in the insulin-induced Akt phosphorylation in the liver.19
Phosphorylation of the IRS-2 in the cascade of insulin activation also influences glycogen synthesis. This phosphorylation inhibits Glycogen Synthase Kinase 3 (GSK-3), which, in normal situations, induces glycogen synthesis and uptake of plasma glucose. In pathological situations, glycogen synthesis is interrupted and glucose is not taken up, leading to the development of IR.
Viral hepatitis and IR
As noted above, several studies have shown that some viral infections promote IR. This association has been established mostly in chronic hepatitis because acute infections promote liver damage rather than metabolic changes because of the duration of the damage. This is why studies have focused on HCV and HBV infections, the most common causes of chronic liver disease. The association between IR or T2DM and hepatitis caused by hepatitis A virus (HAV), cytomegalovirus, or other acute viral infections has not been studied, and we focus the rest of this review on other chronic entities.
HCV infection and IR
Infection with HCV is a leading cause of chronic liver disease. According to the World Health Organization, more than 3% of the world's population (around 170 million people) are infected and about 130 million are at risk of cirrhosis.20 Most infected people (60-80%) develop chronic hepatitis C, which is associated with progressive fibrosis and a 3-9% chance of developing cirrhosis within the next 50 years.4,21
HCV infection is associated with the development of IR and, eventually, T2DM; thus, HCV can be considered a metabolic disease. It has been suggested that HCV core proteins directly damage the pancreatic tissue, inducing P-pancreatic cell dysfunction and, therefore, altering the insulin response to hyperglycemia, which eventually leads to IR.22 Some authors consider that T2DM may be an extrahepatic manifestation of HCV infection.23 In addition, the presence of IR itself predicts a faster progression to fibrosis, cirrhosis, liver failure, and hepatocellular carcinoma, and a poor response to antiviral therapy against HCV.12
In 1994, Allison et al described for the first time the correlation between HCV and T2DM,24 although this relationship was found only in cirrhotic patients with HCV. Later studies reported this association in cirrhotic patients with HBV (although in a lower percentage of patients).25 Subsequent studies found that cirrhosis is not necessary for the development of T2DM because several HCV-infected patients without cirrhosis presented T2DM26-29 (figure 3). These studies also demonstrated that fasting insulin and HOMA-IR levels are higher in HCV-infected patients than in other groups.30

Some authors have suggested that the disturbance of glucose homeostasis is related to the reduced glucose uptake, portosystemic shunting, impaired glucagon metabolism and increase in the production of proinflammatory cytokines such as TNF-α, IL-6, and TGF-β. NS3 and NS5A are structural proteins of the HCV that act as key mediators in the induction of oxidative stress and inflammation. It is thought that NS5A associates with the endoplasmic reticulum (ER) within cells and induces protein phosphatase 2A expression, which stimulates the production of mitochondrial reactive oxygen species (ROS) by releasing calcium from the ER. This protein also activates the Toll-like receptor-4 and the NF-κB pathway, which increase the production of TNF-α and IL-6. By contrast, NS3 has shown to be able to activate NADPH oxidase 2, which then generates ROS. Consequently, both mechanisms promote inflammation and other changes such as the proliferation of hepatic stellate cells, which promote hepatic fibrosis.31
The mechanisms by which HCV produces IR are not entirely clear. It has been suggested that the induction of IR occurs through the serine phosphorylation on the IRS-1 and impairment of the Akt signaling pathway. The mechanisms by which these are altered differ between genotypes, making IR genotype specific. As seen in the pathogenesis of steatosis, where genotype 1 is associated with the metabolic syndrome and genotype 3 is associated with the cytopathic effect of the virus, the IR is also related with the genotype. Although all HCV genotypes can induce IR, some genotypes seem to induce a more severe form of IR. People with genotype 1 or 4 are more prone than those with genotype 3 to develop IR.6 Another study found that the severity of IR is greater in genotype 2a compared with 1.22 Pazienza and colleagues demonstrated that the core protein of genotype 3a downregulates peroxisome proliferatoractivated receptor-γ (PPARγ) and upregulates the suppressor of cytokine signal 7 (SOCS-7), whereas the core protein of genotype 1b activates mTOR and SOCS-3; all these mechanisms cause the phosphorylation of the IRS-1 (figure 4). This discovery shows that the degradation of the IRS-1 in IR development is genotype specific.15,32 Enhanced production of SOCS is related to phosphorylation of the Akt and Phosphatidylinositol 3 Kinase (P13K) pathways, which inhibits the production of GLUT-4 and, therefore, glucose uptake. Furthermore, this family of suppressor cytokines has also been linked to the development of interferon resistance.31

Hepatitis B infection and IR
Even though the introduction of a vaccine against the HBV has diminished the number of cases of HBV infection considerably, the burden of the disease continues to be high worldwide. About 350 million people worldwide are infected and at risk for progression of the disease.33
The reports on the relationship between T2DM and HBV infection are inconsistent. Some authors have found glycemic abnormalities in HBV-infected patients similar to those associated with HCV infection.34 However, others have reported that IR and HBV infection are not related 35,36 and that HBV infection may be protective against the development of IR.37,38 These inconsistencies indicate the need for prospective studies.
The association between HBV and the development of hepatic steatosis is also somewhat controversial. Wang and coworkers affirmed that HBV infection is not related to the development of liver steatosis,36 whereas others have suggested that the accumulation of lipids in the liver occurs through several mechanisms that eventually activate SREBP-1 and PPARγ.39 This last point is interesting because the pathogenesis of HCV-associated IR occurs through the inhibition of PPARγ and, as noted, HBV infection produces the exact opposite effect on PPARγ. Although other mechanisms responsible for IR development have not been studied thoroughly, this last point may explain the purported protective effect of HBV infection.
Other chronic viral hepatitis infections and IR
The other two hepatotropic viruses that may cause chronic hepatitis are hepatitis D virus (HDV) and hepatitis E virus (HEV).
HDV infection has two epidemiological patterns: an endemic pattern in Mediterranean countries and a nonendemic pattern in the USA and northern Europe. The effects of HDV infection are synergistic with those of HBV infection. Most of the time, HDV superinfection promotes an acute event of HBV infection; sometimes both infections can be sustained chronically. No studies have shown an association between HDV alone and IR, although it is known that HDV may accelerate HBV progression and damage.1
Little is known about the pathophysiology of HEV infection. It is known that this infection may cause both acute infection, similar to that of HAV infection, or chronic infection. It was believed that this infection was endemic in developing countries; however, recent studies have shown it to be an emerging disease in developed countries. HEV infection occurs mostly in immunosuppressed patients, especially in patients who have received a transplant, in HIV coinfected patients, and in individuals undergoing chemotherapy for any reason. There are no reports of an association between HEV infection and IR, although as it causes liver damage through the chronic secretion of proinflammatory cytokines, it is possible that it also leads to the development of IR. Further study is needed to confirm this association.40
Conclusion
Mexican population is facing a major health issue because of the increasing prevalence of T2DM. Several studies have shown a high incidence and prevalence, indicating that Mexican population is prone to developing the disease and that the impact has been increasing each year. Because some forms of chronic hepatitis are worsened by IR, chronic liver diseases will become an increasing problem in this population.
Only the chronic forms of viral hepatitis have been studied thoroughly in relation to their association with IR and T2DM. Although some studies have reported an association between HBV infection and IR, it seems more likely that they are not related and that the infection may even provide some protection against the metabolic alterations. Nevertheless, it is clear that prospective studies are needed. It is clear that HCV infection and IR are related, and that their effects are synergistic. IR may complicate HCV infection, possibly by causing interferon resistance (associated with failure of the hepatitis treatment) and progression of liver damage. On the other way around HCV infection may contribute to the development of T2DM and its complications.
The relationship between T2DM and the development of cirrhosis and hepatocellular carcinoma has been increasing worldwide41 and in Mexico. Recent reports show that both, T2DM and cirrhosis are among the three most frequent causes of mortality.42 This is why we encourage all physicians, regardless of their specialty, to consider screening for IR and hyperglycemia in all patients with viral hepatitis infection.
References
1. Dienstang JL, Isselbacher KJ. Acute viral hepatitis, in: Kasper DL, Braunwald E, Fauci AS, et al. Harrison's Principles of Internal Medicine. McGraw-Hill. 16th ed., 2005, New York, EU:1825-1855. [ Links ]
2. Hickman IJ, Macdonald GA. Impact of diabetes on the severity of liver disease. Am J Med 2007;120:829-834. [ Links ]
3. Secretaría de Salud. CENAVECE Epidemiología. Anuario de morbilidad 1984-2008. Available at: http://www.dgepi.salud.gob.mx/anuario/html/anuarios.html. [Consulted: July 15th 2010] [ Links ].
4. Méndez-Sánchez N, Villa AR, Chávez-Tapia NC, et al. Trends in liver disease prevalence in Mexico from 2005 to 2050 through mortality data. Ann Hepatol 2005;4:52-55. [ Links ]
5. Zein NN, Abdulkarim AS,Wiesner RH, Egan KS, Persing DH. Prevalence of diabetes mellitus in patients with end-stage liver cirrhosis due to hepatitis C, alcohol or cholestatic disease. J Hepatol 2000;32:209-217. [ Links ]
6. Moucari R, Asselah T, Cazals-Hatem D, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology 2008;134:416-423. [ Links ]
7. Lecube A, Hernández C, Genesca J, et al. High prevalence of glucose abnormalities in patients with hepatitis C virus infection. Diabetes Care 2004;27:1171-1175. [ Links ]
8. Arao M, Murase K, Kusakabe A, et al. Prevalence of diabetes mellitus in Japanese patients infected chronically with hepatitis C virus. J Gastroenterol 2003;38:355-360. [ Links ]
9. Singal AK, Ayoola AE. Prevalence and factors affecting occurrence of type 2 diabetes mellitus in Saudi patients with chronic liver disease. Saudi J Gastroenterol 2008;14:118-121. [ Links ]
10. Kobashi-Margáin RA, Gutiérrez-Grobe Y, Uribe M, Ponciano-Rodríguez G, Méndez-Sánchez N. Prevalence of type 2 diabetes mellitus and chronic liver disease: a retrospective study of the association of two increasingly common diseases in Mexico. Ann Hepatol 2010; 9:282-288. [ Links ]
11. Knobler H, Schihmanter R, Zifroni A, Fenakel G, Schatner A. Increased risk of type 2 diabetes in noncirrhotic patients with chronic hepatitis C virus infections. Mayo Clinic Proc 2000;75:355-359. [ Links ]
12. Méndez-Sánchez N, Chávez-Tapia NC, Zamora-Valdés D, et al. Hepatobiliary diseases and insulin resistance. Curr Med Chem 2007;14:1988-1999. [ Links ]
13. Nannipieri M, Gonzales C, Baldi S, Posadas R, Williams K, Haffner SM, et al. Liver enzymes, the metabolic syndrome, and incident diabetes. Diabetes Care, 28:1757-1762. [ Links ]
14. Holstein A, Hize S, Thiessen E, Plaschke A, Egberts EH. Clinical implications of hepatogenous diabetes in liver cirrhosis. J Gastroenterol Hepatol 2002;17:677-681. [ Links ]
15. García-Compean D, Jaquez-Quintana JO, Maldonado-Garza H. Hepatogenous diabetes. Current views of an ancient problem. Ann Hepatol 2009;8:13-20. [ Links ]
16. Sindelar DK, Chu CA, Rohlie M, et al. The role of fatty acids in mediating the effects of peripheral insulin on hepatic glucose production in the conscious dog. Diabetes 1997;46:187-196. [ Links ]
17. Boden G, Chen X, Capulong E, Mozzoli M. Effects of free fatty acids on gluconeogenesis and autoregulation of glucose production in type 2 diabetes. Diabetes 2001;50;810-816. [ Links ]
18. Lam TK,Yoshii H, Haber CA, et al. Free fatty acid-induced hepatic insulin resistance: a potential role for protein kinase C-delta. Am J Physiol Edocrinol Metab 2002;283:E682-E691. [ Links ]
19. Wu C, Khan SA, Peng LJ, et al. Perturbation of glucose flux in the liver by decreasing F26P2 levels causes hepatic insulin resistance and hyperglycemia. Am J Physiol Endocrinol Metab 2006;291:E536-E543. [ Links ]
20. World Health Organization. Hepatitis C: 2008. Available at: http://www.who.int/vaccine_research/diseases/viral_cancers/en/index2.html. [Consulted: July 15th 2010] [ Links ].
21. Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 2001;34:809-816. [ Links ]
22. Machado MV, Cortez-Pinto H. Insulin resistance and steatosis in chronic hepatitis C. Ann Hepatol 2009;8:Supplement:S67-S75. [ Links ]
23. Chen HF, Li CY, Chen P, See TT, Lee HY. Seroprevalence of hepatitis B and C in type 2 diabetic patients. J Chin Med Assoc 2006;69:146-152. [ Links ]
24. Allison ME, Wreightt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in cirrhotic population. J Hepatol 1994;21:1135-1139. [ Links ]
25. Mason AL, Lau JY, Hoang N, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology 1999;29:328-333. [ Links ]
26. Antonelli A, Ferri C, Fallahi P, et al. Hepatitis C virus infection: evidence for an association with type 2 diabetes. Diabetes Care 2005;28:2548-2550. [ Links ]
27. Lecube A, Hernández C, Genescá J. Diabetes is the main factor accounting for the high ferritin levels detected in chronic hepatitis C virus infection. Diabetes Care 2004;27:2669-2675. [ Links ]
28. Fraser GM, Harman I, Meller N, Niv Y, Porath A. Diabetes mellitus is associated with chronic hepatitis C but not chronic hepatitis B infection. Isr J Med Sci 1996;32:526-530. [ Links ]
29. Mehta SH, Brancati FL, Sulkowski MS, et al. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med 2000;133:592-599. [ Links ]
30. Kawaguchi T,Yoshida T, Harada M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 up-regulation of suppressor of cytokine signaling 3. Am J Pathol 2004;165:1499-1508. [ Links ]
31. Del Campo JA, Romero-Gómez M. Steatosis and insulin resistance in hepatitis C: A way out of the virus? World J Gastroenterol 2009;15:5016-5019. [ Links ]
32. Pazienza V, Clement S, Pugnale P, et al. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate I through genotype-specific mechanisms. Hepatology 2007;45:1164-1171. [ Links ]
33. Te H, Jensen DM. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liv Dis 2010;14:1-21. [ Links ]
34. Custro N, Carroccio A, Ganci A, et al. Glycemic homeostasis in chronic viral hepatitis and liver cirrhosis. Diabetes Metab 2001;1:476-481. [ Links ]
35. Kumar M, Choudhury A, Manglik N, et al. Insulin resistance in chronic hepatitis B virus infection. Am J Gastroenterol 2009;104:76-82. [ Links ]
36. Wang CC, Hsu CS, Liu CJ, et al. Association of chronic hepatitis B virus infection with insulin resistance and hepatic steatosis. J Gastroenterol Hepatol 2008;23:779-782. [ Links ]
37. Jas CF, Chen CJ, Chiu YH, et al. A population-based study investigating the association between metabolic syndrome and hepatitis B/C infection (Keelung community-based integrated screening study, 10). Int J Obes 2006;30:794-799. [ Links ]
38. Luo B, Wang Y, Wang K. Association of metabolic syndrome and hepatitis B infection in Chinese population. Clin Chim Acta 2007;380:238-240. [ Links ]
39. Kim KH, Shin HJ, Kim K, et al. Hepatitis B virus X protein induces hepatic steatosis via transcriptional activation of SREBPI and PPAR gamma. Gastroenterol 2007,132:1955-1967. [ Links ]
40. Bihl F, Negro F. Chronic hepatitis E in the immunosuppressed: A new source of trouble! J Hepatol 2009;50:435-437. [ Links ]
41. Lagiou P, Kuper H, Stuver S, et al. Role of diabetes mellitus in the etiology of hepatocellular carcinoma. J Nat Can Inst 2000;92:1096-1099. [ Links ]
42. Secretaría de Salud. Sistema nacional de información en salud. [Consulted: July 15th 2010]. Available at: http://sinais.salud.gob.mx/mortalidad. [ Links ]
 Address reprint requests to:
Address reprint requests to:
Dr. Nahum Méndez-Sánchez
Clínica Médica Sur, Unidad de Investigación Biomédica
Puente de Piedra #150, Col. Toriello Guerra
14050 Tlalpan, México DF
E-mail: nmendez@medicasur.org.mx
Received on: October 21, 2010
Acepted on: March 15, 2011
Declaration of conflicts of interest: The authors declare no conflict of interest.














