Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Salud Pública de México
Print version ISSN 0036-3634
Salud pública Méx vol.53 suppl.1 Cuernavaca Jan. 2011
ORIGINALS ARTICLES
Hepatitis C seroprevalence and correlation between viral load and viral genotype among primary care clients in Mexico
Hepatitis C seroprevalence and correlation between viral load and viral genotype among primary care clients in Mexico
Ana I Burguete-Garcia, MD, PhDI; Carlos J Conde-Gonzalez, M Sc, PhDI; Ricardo Jimenez-Mendez, MD, PhDII; Yanet Juarez-Diaz, BScI; Elizabeth Meda-Monzon, BScI; Kirvis Torres-Poveda, PhDI; Vicente Madrid-Marina, MD, PhDI
IInstituto Nacional de Salud Publica, Cuernavaca, Morelos; Mexico
IIFaculty of Paediatrics, University of British Columbia,Vancouver, Canada
ABSTRACT
OBJECTIVE: To measure hepatitis C virus (HCV) sero-prevalence, prevalence, hepatitis risk characteristics frequency, and genotype correlation with viral load among clients attending health care clinics.
MATERIAL AND METHODS: Venous blood samples from l12 226 consecutive consenting adults were collected from January 2006 through December 2009. HCV antibodies were detected by immunoassay. HCV RNA was detected by qRT-PCR and viral genotype was performed by PCR and LIPA test.
RESULTS: The HCV seroprevalence observed was l.5 % (C.I. 95% l.3-l.7), from seropositive individuals 60.9 % reported previous blood transfusion, 28.3% declared to have relatives with cirrhosis, 25.2% had tattoos or piercings, and 6.9% referred to have used drugs. Male gender and transfusion (p<0.00l) were the most frequent hepatitis risk characteristics in the HCV seropositive group. Among seropositive subjects 48.3% presented HCV RNA.The most frequent genotype detected in all geographic areas of Mexico was l (subtype lA, 33%; subtype lB, 21.4%) followed by genotype 2 (subtype 2A, 8.50%). Subjects with genotype 1 had a significant correlation with the highest viral load.
CONCLUSIONS: Our results show that nearly half of seropositive individuals are chronically infected. HCV infection has been shown in this study to be an emerging health problem in Mexico.
Keywords: HCV; seroprevalence, genotypes; viral load; Mexico.
RESUMEN
OBJETIVO: Medir la seroprevalencia y prevalencia del virus de hepatitis C (VHC), la frecuencia de caracteristicas de riesgo y la correlacion genotipica con la carga viral en sujetos asistentes a clinicas de medicina familiar.
MATERIAL Y METODOS: muestras de sangre venosa se colectaron de l12 226 adultos, previo consentimiento informado, de enero 2006 hasta diciembre 2009, para la deteccion de anticuerpos contra VHC por ELISA. La deteccion de RNA-VHC y el genotipo viral se realizo mediante qRT-PCR.
RESULTADOS: La seroprevalencia de VHC fue l.5 % (C.I. 95% l.3-l.7), 60.9% reportaron transfusion sanguinea previa, 28.3% dijo tener familiares cercanos con cirrosis, 25.2% tenian tatuajes o piercing y 6.9% refirio ser usuario de drogas intravenosas. El ser hombre, el antecedente de transfusiones y el uso de drogas (p<0.00l), fueron los factores con mayor frecuencia en el grupo VHC seropositivo. La prevalencia del RNA-VHC en seropositivos fue de 48.3%. El genotipo mas frecuente en todas las areas geograficas de Mexico fue el l (subtipo lA, 33%; subtipo lB, 21.4%) seguido por el genotipo 2 (subtipo 2A, 8.50%). Se observó una correlación positiva de 51% con la carga viral más alta y el genotipo viral 1A.
CONCLUSIONES: Nuestros resultados muestran que cerca de la mitad de individuos seropositivos están infectados crónicamente. Esta infección debe considerarse como un problema emergente de salud pública en México.
Palabras clave: VHC; seroprevalencia; genotipos; carga viral; México.
The chronic infection caused by the virus of hepatitis C (HCV) represents a recognized problem of public health world-wide.1 It is estimated that 300 million people currently have chronic infection by HCV in the world. This infection annually causes the death by its complications to approximately 1,2 million people in the world.2 The onset of disease is usually insidious, with anorexia, vague abdominal discomfort, nausea and vomiting, fever and fatigue, progressing to jaundice in about 25% of patients, less frequently than hepatitis B.3 Of those exposed to HCV, about 40% recover fully, but the remainder, whether they have symptoms or not, become chronic carriers. The chronic infection can progress to cirrhosis (20%) along with its complications like ascitis, encephalopathy, bleeding of esophagic varices and hepatocellular carcinoma.4
For that reason the early detection and treatment of the infection are of extreme importance to reduce the morbidity and mortality in the affected population. The main problem for the diagnosis and the treatment of the chronic infection caused by the HCV is the genetic virus heterogeneity, it is an enveloped RNA virus, classified as a separate genus (Hepacivirus) within the Flaviviridae family, and presents a high rate of mutations, which has given rise to different viral genotypes. Nowadays, 6 genotypes are known that are distributed with different proportions between the infected populations. In the United States it has been observed that genotype 1 with approximately 75% of the cases predominates, followed by genotypes 2/3 with approximately 10% each, in relation to the total of cases.5, 6 The infection by HCV has a world-wide distribution whose frequency varies according to regions or countries. In the majority of the developed countries the prevalence of the infection in the population level varies between 1% and 2%.7,8
In Mexico, the only published research effort, a work with a population base approach in which the average prevalence of the infection by HCV has been considered, is the National Survey of Health of the year 2000.9 The results of this study showed that the global prevalence of antibodies against HCV in adult general population, of both sexes, was 1,4%. This frequency corresponds to 700 000 people in the Mexican Republic. Although the frequency of the infection in the population level in Mexico is low, if we compare it with the frequencies reported in other countries, it is necessary to emphasize that only 37% of the seropositive individuals to HCV had active infection, since they were positive to the RNA-PCR test, which identifies HCV RNA.9 It was estimated then that 259 000 people are chronic carriers of the virus in Mexico, and which possibly will evolve towards the complications of chronic hepatitis, particularly cirrhosis and hepatocellular carcinoma.
Recently, Santos-Lopez et al .10 conducted a systematic review using several free-access databases to explore the prevalence of HCV infection in the Mexican population. Sixty-eight works fulfilled the search criteria. From these, 44 studies involved asymptomatic subjects and 28 involved patients or high-risk subjects. Prevalence of blood donors (6 955 558 persons) ranged from 0.0% to 2.05%, with 7/32 studies reporting values >1%, whereas prevalence of non-donor asymptomatic subjects (28 528 persons) was from 0.0% to 2.7%, with 7/11 studies reporting values >1%, and medical personnel from 0.0% to 2.08% (1,227 persons), with 4/11 studies reporting values >1%. Prevalence of patients with chronic hepatic disease ranged from 6.7% to 77%. The most prevalent genotype was 1 (30.0%-87.5%), of which subtype 1b is the most frequent (11.9%-61.9%). The main risk characteristics were blood transfusion and unprotected sex or having multiple sex partners. The prevalence in the Mexican population seems to be in accordance with that previously estimated by the World Health Organization (1%-2.5%).2 The aim of this study was measuring HCV sero-prevalence, prevalence, risk characteristics frequency, and its genotype correlation with viral load among clients of family medicine clinics.
Materials and Methods
This transversal study included 112 226 consecutive consenting female and male adults, who were seeking attention at family medicine clinics for multiple causes but different to any kind of liver disease, all subjects were between 18 and 69 years old. This project was carried out in medical units of the first and / or second level of public health institutions, selected by a logistical and geographical convenience. Samples were collected from 19 states of Mexico, we used a geographic distribution adapted from Esquivel G. (2000), which included four regions: North, Center, Pacific / South and Distrito Federal/Estado de Mexico.11 National Institute of Public Health Research and Ethics Committees approved the study, and all participants gave an informed signed consent after explaining the purpose of the study and highlight the importance of early identification of chronic carriers of HCV infection for treatment. The main criterion for selecting individuals for the study was a history of at least one of the following risk characteristics for HCV infection: a history of blood transfusion before 1995, the presence of unsafe sex practice, injecting drug use, the presence of relatives with hepatitis C or liver cirrhosis and the presence of tattoos and body piercings, excluding the common practice of drilling ears in women, which is a normal cultural practice in Mexico. All the individuals that had previous diagnosis of hepatitis or liver disease were excluded from the study.
From all patients who agreed to participate in the study, 10 milliliters of venous blood was extracted. The collected samples of blood were centrifuged to obtain serum and enzyme immunoassay (ELISA) test was performed at our facilities. We performed the determination of antibodies (IgG) anti-HCV by ELISA. This is a validated commercial assay; it is performed on automated equipment (Johnson and Johnson Company). All samples that were positive by ELISA test were subject to a confirmatory test by searching in serum HCV RNA by qualitative RT-PCR test COBAS AMPLICOR.
Genotyping (LIPA). HCV subtyping was accomplished by amplifying and sequencing a 339-bp amplicon of the NS5b region. A seminested PCR was performed with primers NS5-1 (sense; 5_-TAT-GAYACC-CGY-TGC-TTT-GAC-3_) and NS5-2 (reverse; 5_-GAG-GAG-CAA-GAT-GTT-ATC-AGC-TC-3_) for primary amplification and primers NS5-1 and NS5-3 (reverse; 5_-GAA-TAC-CTG-GTC-ATA-GCC-TCCG-3_) for secondary amplification. Subtyping was also carried out with type-specific primers, as described previously.12
We performed a statistical analysis to calculate the frequency distributions of the variables used in the study, the frequency of HCV antibodies obtained by ELISA, and the frequency of the presence of viral RNA detected by RT-PCR test. The relationship of the frequencies of the markers of HCV infection and the above risk characteristics reported by the respondents was evaluated in the ELISA positive group and in the PCR positive subsample. Finally to evaluate our hypothesis we performed a direct correlation analyses between viral load and viral genotype.
Results
This study included 112 226 consecutive consenting female and male adults, who were seeking attention at family medicine clinics. The 19 states used for this study were divided for region North: Baja California, Sinaloa, Sonora, Chihuahua, Coahuila, Nuevo Leon, and Tamaulipas; for region Center: Aguascalientes, Queretaro, Hidalgo and Puebla; for region Pacific/ South: Nayarit, Colima, Jalisco, Veracruz, Yucatan and Quintana Roo; and for the final region: Distrito Federal/Estado de Mexico. All subjects were between 18 and 69 years old, with a mean age of 42.16 (SD 12.75) years. (Table I)

The HCV overall sero-prevalence observed in the study population was 1.5 % (C.I. 95% 1.3-1.7), with a mean age of 43.6(C.I. 95% 43-44.2). The frequencies of HCV antibodies by geographic region were the following: North 1.65% (C.I. 95% 1.57-1.72), Center 1.55% (C.I. 95% 1.48-1.62), Pacific/South 0.81% (C.I. 95% 0.76-0.86) and Distrito Federal/Estado de Mexico 1.59% (C.I. 95% 1.52-1.67). In our study the positive seroprevalence was 1.5% (N=1 681). Male gender predominated among HCV positive sero-prevalence, in the same group with positive seroprevalence we observed that 60.9% had received a blood transfusion, 28.3% declared to have relatives with cirrhosis, 25.2% had tattoos or piercings, only 6.9% referred to have used drugs. The most frequent risk characteristics were male gender and blood transfusion (p<0.001). (Table I)
The prevalence of HCV RNA detected by RT-PCR among seropositive persons was 48.3%. All of these currently infected people were referred to medical care. In the comparative analyses between HCV RNA positive and negative we did not find significant differences in drug use, blood transfusion, risky sexual practices. Nevertheless, male gender and family history of cirrhosis had a bigger frequency in the HCV RNA positive group when it was compared with the HCV RNA negative group (53.75 vs. 46.25%, p value 0.002 and 58.42% vs. 41.58%, p value: 0.03; respectively). The distribution of hepatitis risk characteristics and viral genotypes detected in the positive sero-prevalence subsample (N=1681, 1.5%) is shown in table II.
As an exploratory analysis, in one hand we evaluated the prevalence of viral genotype in all geographic areas of Mexico where the most frequent genotype detected in all geographic areas of Mexico was genotype 1/subtype 1A (33%) followed by genotype 1/subtype 1B (21.4%), and genotype 2/subtype 2A (8.50%). On the other hand we evaluated the prevalence of viral genotype by hepatitis risk characteristics, where the viral genotype 1 was the most frequent in the individuals with tattoos or piercings, and among individuals who declared having used drugs (Pearson's chi2(1)=11.1239, Pr = 0.001, Pearson's chi2(1) = 8.7961, Pr =0.003, respectively). No significant difference was observed for blood transfusion, family history of cirrhosis and risky sexual practices. Also in our study population we observed that blood transfusion was the most frequent hepatitis risk factor in the North area (50.77%) and less frequent in Pacific and South areas (9.37%). Drug use, risky sexual practices, and tattoos and piercings were the most frequent risk characteristics in the Center area. (Data not shown)
Finally, to evaluate our hypothesis about direct correlation between observed viral load and viral genotype, we found a significant linear positive correlation for the highest viral load and viral genotype 1 with a R2 of 80%. (Figure 1)
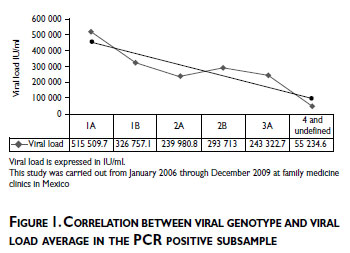
Discussion
In Mexico, several studies have been conducted to determine HCV seroprevalence, among different populations; this has been matter of relatively extensive research. However, data are scarce regarding population based 10,13-1 5 Therefore, we re-addressed this problem and conducted this study. The findings about HCV seroprevalence, virus prevalence and genotyping contribute to the external validity of these results, because they correlate with previous studies that describe the same risks characteristics for hepatitis C.8,15 However, to have relatives with cirrhosis is a new factor that had not been observed previously in a Mexican study.
Regarding the geographic distribution of HCV infection in this study, it reinforces what has been recorded in Mexico at various times by several authors.9,10,13,16,17 This is even though there have been different study designs where regions North, Center, and the greater metropolitan area in the country are the main focus of public health attention to care for prevention of disease and opportune diagnosis and treatment of affected people.
Acknowledging the limiting effect of indeterminate genotyping results, the most frequent genotype detected in the population assessed was genotype 1/ subtype 1A followed by genotype 1/subtype 1B, and genotype 2/subtype 2A. These results are in agreement with those previously reported.16,1 7 Also, we observed a substantial correlation between highest viral load and HCV genotype 1. The higher correlation between viral load and genotype 1, may contribute to the poor response to treatment against HCV chronic infection.18 These results could help to monitoring of new strategies of treatment especially among patients with genotype 1 and contribute to diminish the economic burden of hepatitis C in Mexico.18
The notification network established during this investigation is noteworthy to mention, as it has been structured with physicians, who are at primary care clinics, with a role of "sentinel physicians". In order to complement the network, the "local observatories" or "sentinel areas" (services of the Family Medicine Units) are the places where the information notified by the sentinel physicians is captured and sent to the "central registry facility" (National Institute of Public Health of Mexico) to process the information of the local observatories. The three levels integrate the sentinel notification network.
Thus, the components for the application of the sentinel event and the network of notification are the following:19,20 active participation of the services of the primary care units; determination of a situation of clearly identifiable disease in its users; definition of the determining processes; availability of a system of monitoring for the harvesting of valid information; its analysis and diffusion, and implementation of an effective strategic intervention.
Despite the non-homogeneous distribution of our sample and lack of representativity of the samples collected from 19 Mexican states, our results contribute to have a better knowledge about seroprevalence and genotype distribution of HCV in a sizable sample of the Mexican population.
In the present sentinel surveillance study of hepatitis C virus, an additional interest was the identification of users of the services of Family Medicine Units, that have at least a known risk factor of infection by HCV, to evaluate the frequency of the infection in this group and to detect those individuals that present with chronic infection to give opportune treatment and to prevent their evolution towards cirrhosis or hepatocellular carcinoma.
The detection of hepatitis C has been greatly impacted by mandatory screening of blood donors in most countries in the world,21 although intravenous drug use continues to be a major source of infection, including Mexico,22,23 where additionally the main risk characteristics are blood transfusion and probably to have relatives with cirrhosis. Public education regarding the risks of exposure to infecting paraphernalia as well as household items such as razors is necessary in the continuing effort to curb this disease.
Finally, a public health message derived from this study and other recent ones9,17 is about the awareness due to HCV infection in the Mexican population requiring prompt intervention at the community level, to control and prevent further evolution of the problem. In ample terms, specific measures might include sustained quality surveillance of blood donation, availability in the public health sector of anti-viral treatment for infected people, an effective program for the provision of sterile syringes and needles among drug users, screening of pregnant women with risk characteristics for HCV acquisition to avoid vertical transmission of the virus and even reinforcing proper condom use for people with high risk sexual practices. Therefore, the early detection of the infection and the appropriate therapy are of extreme importance to reduce viral transmission, morbidity and mortality in the afflicted population.
Acknowledgements
To Roche Pharma for providing HCV diagnostic laboratory reagents used in this study.
References
1. Mendez-Sanchez N, Uribe M. Consenso Nacional sobre Hepatitis C. Conclusiones. Rev Invest Cliln 2002;54:559-568. [ Links ]
2. WHO . Global surveillance and control of hepatitis C. Report of a WH O Consultation organized in Collaboration with the Viral Hepatitis Prevention Board,Antwerp, Belgium. J Viral Hepatol 1999; 6:35-47. http://www.who.int/csr/disease/hepatitis/whocdscsrlyo2003/en/print.html (consulted on November 2, 2010). [ Links ]
3. CDC. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR 1998;47(RR19):1-39. [ Links ]
4. EASL International Consensus Conference on Hepatitis C. Consensus Statement. J Hepatol 1999;31:3-8. [ Links ]
5. Bukh J, Miller RH, Purcell RH. Genetic heterogeneity of hepatitis C virus: quasispecies and genotypes. Semin Liver Dis 1995;15:42-63. [ Links ]
6. Flamm SL. Chronic hepatitis C virus infection. JAMA 2003;289:2413-2417. [ Links ]
7. Alter MJ. Epidemiology of hepatitis C in the West. Semin Liver Dis 1995;15:5-14. [ Links ]
8. Di Bisceglie AM. Hepatitis C. The Lancet 1998;351:351-355. [ Links ]
9. Valdespino JL, Conde-Gonzalez CJ, Olaiz-Fernandez G, Palma O, Kershenobich D, Sepulveda J. Seroprevalencia de la hepatitis C en adultos de Mexico: jun problema de salud publica emergente? Salud Publica Mex 2007;49(Suppl. 3):S395-403. [ Links ]
10. Santos-Lopez G, Sosa-Jurado F,Vallejo-Ruiz V, Melendez-Mena D, Reyes-Leyva J. Prevalence of hepatitis C virus in the Mexican population: A systematic review. J Infect 2008;56:281-290. [ Links ]
11. Esquivel, G. Geografia y Desarrollo Economico en Mexico. Colegio de Mexico, Mexico D.F. 2000:9-14. [ Links ]
12. Cantaloube JF,Venault H, Zappitelli JP, Gallian P,Touinssi M,Attoui H, et al. Molecular analysis of HCV type l to 5 envelope gene: application to investigations of post-transfusion transmission of HCV. Transfusion 2000;40:712-717. [ Links ]
13. Mendez-Sanchez N, Motola-Kuba D, Zamora-Valdes D, Sanchez-Lara K, Ponciano-Rodriguez G, Uribe-Ramos MH, et al. Risk characteristics and prevalence of hepatitis virus B and C serum markers among nurses at a tertiary-care hospital in Mexico City, Mexico: a descriptive study. Ann Hepatol 2006;5:276-280. [ Links ]
14. Benitez-Arvizu G, Cortez-Gomez R, Novelo-Garza BA, Malagon-Martinez A, Guerra-Marquez A,Alvarado-Maldonado M del C, et al. Prevalence of hepatitis C virus in the blood bank at Centro Medico Nacional La Raza. Rev Med Inst Mex Seguro Soc 2006;44:227-233. [ Links ]
15. Garcia-Montalvo BM, Galguera-Colorado PL. Distribution of hepatitis C virus genotypes, risk characteristics and liver disease in patients from Yucatan, Mexico. Ann Hepatol 2008;7:345-349. [ Links ]
16. Idrovo AJ, Fernandez JA. Which is the real genotype distribution of hepatitis C virus infection in Mexico? Ann Hepatol 2008;7:390-391. [ Links ]
17. Jimenez-Mendez R, Uribe-Salas F, Lopez-Guillen P, Cisneros-Garza L, Castaneda-Hernandez G. Distribution of HCV genotypes and HCV RNA viral load in different regions of Mexico. Ann Hepatol 2010;9:33-39. [ Links ]
18. Gish RG, Arora S, Rajender-Reddy K, Nelson DR, O'Brien C, Xu Y, et al .Virological response and safety outcomes in therapy-naive patients treated for chronic hepatitis C with taribavirin or ribavirin in combination with pegylated interferon alfa-2a: a randomized, phase 2 study. J Hepatol 2007;47:51-59. [ Links ]
19. CDC. Guidelines for evaluating surveillance systems. MMWR 2001;50:1-35. [ Links ]
20. Rutstein DD,Mullan RJ,Frazier TM,Halperin WE , Melius JM, Sestito JP. Sentinel health events (Occupational): a basis for physician health surveillance. Am J Public Health 1983;73:1054-1062. [ Links ]
21. Te HS, Jensen DM. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis 2010;14:1-21. [ Links ]
22. White EF, Garfein RS, Brouwer KC, Lozada R, Ramos R, Firestone-Cruz M,et al, Prevalence of hepatitis C virus and HIV infection among injection drug users in two Mexican cities bordering the U.S. Salud Publica Mex 2007;49:165-172. [ Links ]
 Address reprint requests to:
Address reprint requests to:
Dr.Vicente Madrid Marina
Av. Universidad No. 655 Colonia Santa Maria Ahuacatitlan
62100, Cuernavaca, Mor. Mexico.
E-mail: vmarina@insp.mx
Received on: February 23, 2011
Acepted on: June 27, 2011
Declaration of conflicts of interest: The autors declare no conflicts of interest.














