Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Salud Pública de México
versión impresa ISSN 0036-3634
Salud pública Méx vol.52 no.6 Cuernavaca nov./dic. 2010
ARTÍCULO ORIGINAL
Short-term risk of cervical intraepithelial neoplasia grades 2 and 3 for women with normal cytology and human papillomavirus infection
Riesgo a corto plazo de lesiones intraepiteliales cervicales grados 2 y 3 en mujeres con citología vaginal normal e infección por el virus del papiloma humano
Gustavo Hernández-Suárez, MD, MScI; Natasha Ortiz, MDI; Mauricio González, MDI; Nubia Muñoz, MD, MPHI
I Instituto Nacional de Cancerologí-a (INC), Bogotá, Colombia.
ABSTRACT
OBJECTIVE. To assess the risk of cervical intraepithelial neoplasia grades 2, 3 or higher (CIN 2/3+) for women with normal cytology and concurrent high-risk human papillomavirus infection (HR-HPV).
MATERIAL AND METHODS. We examined 2 200 women every 6 months for an average of 9 years. Cervical smears and samples for HPV DNA were obtained at each visit. Absolute risk of subsequent CIN2/CIN3+ was estimated using the Kaplan-Meier method.
RESULTS. The absolute risk of CIN2/CIN3+ among HR-HPV-positive women with normal Pap smear results was 1.06% (95%CI, 0.57–2.20), 5 times higher the risk among all women with normal Pap smears (0.20%; 95%CI, 0.12–0.32) but 7 times lower than that for women with HR-HPV infection and LSIL (7.24%; 95%CI, 3.78–15.2).
CONCLUSION. Short-term absolute risk of CIN2/3+ after a normal Pap smear with concurrent HR-HPV infection is low (~1%), suggesting that the HR-HPV test has limited utility in short-term clinical decision-making for women with normal cytology.
Key words: cervical intraepithelial neoplasia; human papilloma virus; risk assessment; Colombia
RESUMEN
OBJETIVO. Evaluar el riesgo a corto plazo de neoplasia intraepitelial cervical de alto grado (CIN2/CIN3+) en mujeres con citologí-a cervicouterina normal e infección por virus del papiloma humano de alto riesgo (HR-HPV).
MATERIAL Y MÉTODOS. Cohorte prospectiva de 2200 mujeres evaluadas cada seis meses durante 9 años en promedio. En cada visita se tomó muestra cervical para extendido y detección de HPV DNA. El riesgo absoluto de CIN2/CIN3+ a la siguiente visita fue calculado utilizando el método de Kaplan-Meier.
RESULTADOS. En mujeres con citologí-a normal e infección concomitante por HR-HPV el riesgo absoluto de presentar CIN2/CIN3+ fue de 1.06% (95%CI, 0.57-2.20). Este riesgo fue cinco veces mayor al observado en todas las mujeres con citologí-a normal (0.20%; 95%CI, 0.12-0.32) pero siete veces menor que el observado en mujeres con lesiones intraepiteliales escamosas de bajo grado con infección concomitante (7.24%; 95%CI, 3.78-15.2).
CONCLUSIÓN. El riesgo absoluto de CIN2/3+ a corto plazo luego de una citologí-a normal e infección por HR-HPV es baja (~1%), sugiriendo que, a corto plazo, la prueba de HR-HPV tiene utilidad clí-nica muy limitada en mujeres con citologí-a normal.
Palabras clave: neoplasia intraepitelial del cuello uterino; virus del papiloma humano; medición de riesgo; Colombia
Unequivocal and extensive evidence supports the conclusion that infection with certain types of human papillomavirus (HPV) –such as high-risk HPV (HR-HPV) types– is a necessary but not a sufficient cause of cervical intraepithelial neoplasia and cervical cancer worldwide.1,2 This knowledge has had enormous impact on health care, including the use of HR-HPV testing in the triage of atypical squamous cells of undetermined significance (ASCUS),3 which has significantly reduced colposcopy referrals without losing the sensitivity to detect cervical cancer. Further, emerging evidence shows that HR-HPV detection is a good alternative to cytology as a primary screening test.4 High expectations for the potential of HPV screening have led certain countries to introduce it into their screening programs –in addition to cervical cytology– despite the lack of consensus on the clinical management of women with normal Pap smear and HR-HPV infection.5-7 Results from recent randomized controlled trials comparing cytology alone to the use of HPV tests in parallel with cytology have shown that the latter detects more precancerous lesions of the cervix than screening with cytology alone.8-10 In addition, a recent randomized controlled trial conducted in Finland –in which the HPV assay was used as primary screening, followed by triage with cytology– has shown that this sequential screening strategy, when compared with the traditional screening based on cytology, not only increases the detection of precancerous lesions but also the specificity.11 In Mexico, results from a recent community-based study12 have shown that the introduction of HR-HPV testing can also improve cervical cancer prevention in developing countries.
Few prospective studies have assessed the absolute risk of precancerous cervical lesions among women with normal Pap smear and positive HR-HPV results.13-15 Studies have previously determined long-term risks (i.e., 5–10 years) but not the short-term or immediate risks (i.e., the current risk or the risk at next visit), which is a frequent question from patients seen in clinical practice. Available case control studies do provide an answer to this question, reporting increased odds of high-grade squamous intraepithelial lesions (HSIL) immediately after an HR-HPV infection among women with normal Pap smear results.16,17 However, odds ratios (as a relative measure of effect) are not informative regarding the absolute risk for defined exposure. Moreover, such values are not always easy to interpret, making their application in clinical practice fairly difficult.18 In fact, high odds ratios do not necessarily mean high absolute risks; thus, for the purpose of clinical decision-making it would be useful to identify the absolute risk of CIN2/CIN3+ incidence at the next visit for regularly screened women with an HR-HPV infection and normal Pap smear. In other words, we will try to answer the following question: If a woman has a normal cytology and an HPV-HR positive result, what is the risk of having a CIN2/CIN3 in the next visit, 6-12 months later?
In this paper, we assessed the absolute risk of subsequent CIN2/CIN3+ among women attending scheduled screening visits, based on their Pap smear results and HPV status. In addition, we assessed the association of known risk cofactors of cervical cancer among women with HR-HPV infection and normal Pap smear results.
Material and Methods
Detailed methods of recruitment and data collection have been described elsewhere.19 In brief, in 1992 the Colombian National Institute initiated a national cervical cancer control program. We identified four health districts in Bogota with low-income neighborhoods and no previous access to an organized cervical cancer screening program. Between 1993 and 1995, using the recruitment files of this program, we invited the first 2000 identified women aged 18 and over to participate in a population-based cohort study that aimed to study the natural history of HPV infection. In addition, we invited 200 girls (selected consecutively) ages 13-17 seen at family planning clinics in the same area to participate in the study.
Ethical approval was obtained from the Review Board of the National Cancer Institute of Colombia. Written informed consent was obtained from each patient prior to participation in the study. Follow-up examinations included scheduled visits every 6 months for up to 10 years. At each visit, a gynecological examination, a Pap smear test, and an epidemiological questionnaire on cervical cancer risk factors was administered to each participant. Sixty-one women refused to participate or were considered ineligible (because of mental illness, hysterectomy, or a history of cervical cancer). The recruitment phase of the study lasted until the end of 1994, and the follow-up period ended in 2004.
Testing for HPV was conducted using a standard GP5+/GP6+ polymerase chain reaction enzyme immunoassay (PCR-EIA) as previously described.19 Briefly, HPV-positive samples by GP5+/GP6+ PCR were subjected to EIA-HPV group-specific analysis using cocktail probes for high-risk and low-risk HPV types.20 The high-risk HPV cocktail probe consisted of oligoprobes for HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68. HPV infection was analyzed based on the epidemiological categorization proposed by Muñoz et al. for high-risk and low-risk HPV types.21 The β-globin gene was amplified in 94% of the sample specimens, and the HR-HPV detection rate was 11%.
Pap smears were read by two expert pathologists using the Bethesda system22 and rated as follows: normal, atypical squamous cells of undetermined significance (ASCUS), atypical glandular cells (AGUS), and low- or high-grade squamous intraepithelial lesions (LSIL or HSIL, respectively). Women underwent colposcopy (and further biopsy to visualize suspected lesions) if abnormalities were detected in the Pap smear. The same two pathologists read the biopsies and classified them as follows: normal; cervicitis; cervical intraepithelial neoplasia (CIN) grade 1, 2, or 3, or; invasive cervical cancer (ICC). Pathologists and clinicians were always blinded to the HPV results.
Statistical analysis
The unit of analysis was pairs of consecutive Pap smear test results. Information regarding the exposures and outcomes of interest were obtained from the first (index) and the second (follow-up) smears, respectively. Women with only one Pap smear result, with pairs of Pap smears without an HPV result at the index visit, or with a time gap of 3 years or more between two visits were excluded from the analysis. Our main composite endpoint was CIN2, CIN3, invasive carcinoma, and HSIL without histological confirmation. HSIL cases without histological confirmation were included due to the high specificity of Pap smear results and the low sensitivity of colposcopy, since a recent report has indicated that colposcopy with only one biopsy misses at least 30% of CIN3-positive cases.23
The Kaplan-Meier method and 95% jackknife confidence intervals (95%CI) for clustered observations were used to estimate the absolute risk based on the index Pap smear results according to age group and HR-HPV status. A longitudinal approach with changing exposure status24 was considered, allowing several pairs of visits by the same women (clusters) to be analyzed as separate observations, each with a different HPV status at index visit. To assess the association of relevant HPV cofactors, we calculated adjusted ORs that fitted a random effects model for correlated data25 using Poisson regression. The HPV cofactors related to cervical cancer that we included in the analysis were age (<30 and ≥30 years), parity (<3 and ≥3 pregnancies), oral contraceptive use, smoking status (ever/never smoked) and the time difference between subsequent visits. Only pairs of Pap smears with no more than a 3-year gap between the index and follow-up visits were considered for this analysis. All statistical analyses were performed using STATA (Statistical Software: Release 9.0., Stata Corporation, College Station, TX: Stata Corporation).
Results
Of the 2 139 women who were eventually included in the study, 261 came only for the initial visit and did not return for further follow-up; they were therefore excluded from this analysis. Of the remaining 1 878 women, 18 417 consecutive pairs of Pap smear results were included, 13 284 of which had a valid HPV result at the index visit, while 318 pairs had to be excluded due to a gap of 3 years or more between visits. Thus, a total of 12 949 (70%) pairs –clustered in 1 409 women– were left for analysis. The average and median ages of the women at the time of the index visit were 46 years and 44 years, respectively. The mean and median durations between two visits were 10 and 6 months, respectively. By 2003, 10 years after the first enrolment, 75% percent of women with more than 1 visit were still attending the scheduled visits.
Thirty-six incident cases (9 CIN2, 16 CIN3, 3 invasive carcinoma, and 8 HSIL) were observed during 11717 p-years of follow up, of which 21 (7 CIN2; 7 CIN3; 2 invasive cancer and 5 HSIL) were detected following a visit with normal Pap smear results.
Absolute risk of CIN2/CIN3+ among women with normal Pap smear results and concurrent positive HR-HPV infection was 1.06% (95%CI: 0.57–2.20), which is 5 times higher than the CIN2/CIN3+ risk observed among all women with normal Pap smear results ( 0.20; 95%CI, 0.12–0.32) but less than half the risk among HR-HPV-positive women with LSIL abnormality in the Pap smear (3.00; 95%CI, 1.66- 5.97) (Table I). The absolute risk (1.84%) related to HPV16 in women with normal Pap smear results was 9 times higher than that for all women with normal Pap smear results and lower than that for women with LSIL (20.54%) and ASCUS (9.39%) results. No risk difference for CIN2/CIN3+ was evident with age. The risk of CIN2/3 was constant within 6-month intervals of time (data not shown).
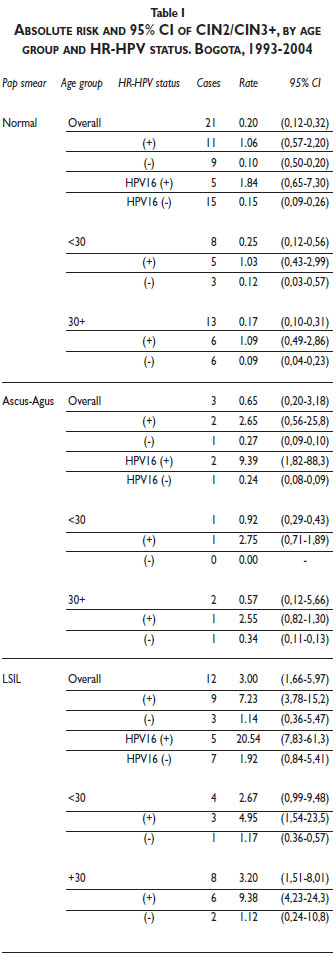
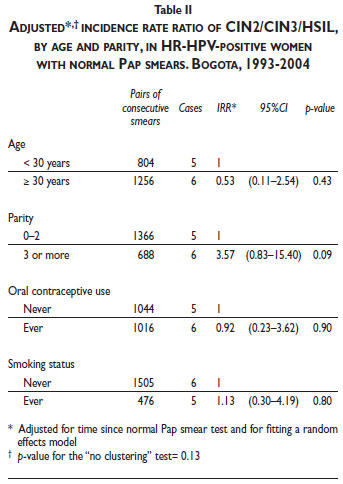
The multivariate analysis of cofactors associated with CIN2/CIN3 risk among women with normal Pap smear results and concurrent positive HR-HPV results showed evidence of a borderline association with parity (≥3 pregnancies; IRR 3.57; 95% CI, 0.83–15.40; p = 0.09), including after taking clustering into account (p = 0.13) (Table II).
Discussion
To our knowledge, this is the first report that focuses on the short-term (immediate) risk of CIN2/CIN3+ for women followed periodically for more than 5 years. While previously published results exist on incidence and determinants of HPV infection26 and on persistence and risk of high-grade cervical lesions in this cohort27 –providing insight into long-term CIN2/CIN3 risk in our population– our current analysis reveals the magnitude and differences in short-term CIN2/CIN3 risk among women with differing Pap smear results and HR-HPV status.
The risk of CIN2/CIN3+ for women with normal Pap smear results and even those with concurrent positive HR-HPV status (cytology–ve/HR-HPV+ve) was low as compared to that observed among women with mild abnormalities. This result may imply that establishing HR-HPV status for women with normal cytology who were followed for 12 months or less is not as useful as it is for women with ASCUS or LSIL cytology.
According to our results, HPV-HR testing of women with a normal Pap smear result requires 125 screenings (NNS= /Risk difference of intervention * 100) to detect CIN2/CIN3+ cases. This value is much higher compared to the NNS of HPV-HR testing observed for women with ASCUS or LSIL (50 and 25, respectively). Previous studies have already recognized that the positive predictive value of a single Pap-negative, HPV-positive cotest for CIN3 or higher remains less than ideal.28
The risk of CIN2/CIN3+ related to HPV 16 infection is the highest among women with normal and abnormal cytology. This result agrees with the EUROGIN24 recommendation of using a single HPV 16 test as an alternative, to increase the specificity of the HR-HPV clinical test (i.e., Hybrid Capture II). However, the low short-term absolute risk observed among women with normal cytology and concurrent HPV 16 infection (1.84%) contrasts with that observed among women with LSIL (20.54%), suggesting a potential limitation of the short-term clinical utility of HPV-HR testing for women with a normal Pap smear result.
A recent clinical trial showed that CIN2+ lesions –which may spontaneously regress among women aged 25-34 years– may be over diagnosed if such women are immediately referred to colposcopy after a positive HR-HPV result.29 In the case of older women, the results still remain inconclusive. Data from the follow-up phase of the same trial, which is currently underway, is expected to provide a direct estimate of regression rates at different ages. Despite the fact that lesions found in the 35 and older age group are expected to be potentially progressive, our results show that the absolute risk of CIN2/3 in the short-term does not differ with age. This is in agreement with the accumulating evidence that highlights the relevance of HR-HPV persistence to CIN2/CIN3+ etiology and progression.27,30 Thus, clinical decision-making based on a single HR-HPV-positive result must be revaluated. Clinicians should bear in mind that the number of women presenting with concurrent normal Pap smear and positive HR-HPV results will probably increase in the near future. A conservative approach, in accordance with current evidence,28 would be to retest the HPV status after 1 or 2 years of women with normal cytology and concurrent HR-HPV positive result.
Higher absolute risk of CIN2/CIN3+ in women with lesser abnormalities agrees with the natural progression of squamous intraepithelial lesions. Unfortunately, the low number of events does not let us draw inferences regarding the differences in risk between women with ASCUS and LSIL Pap smear results. Other studies have shown overall higher rates of progression in women with LSIL as compared to women with ASCUS, despite similar rates of HR-HPV infection.31
Parity was the only cofactor that influenced the incidence risk of CIN2/CIN3+ for women in this population with normal Pap smears. Women with normal Pap smear results and more than two pregnancies presented a higher risk of detecting CIN2/CIN3 after an HR-HPV-positive test. Pregnancy has been shown to increase the exposure of the transformation zone on the exocervix, escalating the risk of repeated HPV infections. Recently, a large pooled analysis of multicentric case-control studies on pregnancy and its association with cervical cancer demonstrated that numerous pregnancies are related to invasive cervical cancer and early pregnancies are related to CIN3+ lesions; thus, parity is a relevant cofactor that can influence the difference in the cervical cancer risk between developed and developing countries.32
Although our results are obtained from a population-based study, we acknowledge that they may not be generalizable to all populations worldwide. In fact, the likelihood of a concurrent HR-HPV-positive result and a normal Pap smear test was higher among our study population of women older than 30 years (7.4%) in comparison with other population-based studies,28 reflecting differences in HR-HPV infection dynamics across populations and thus affecting the positive predictive value of HR-HPV testing.
Our study has several strengths, including the relatively large sample size, the broad age range covered, the low proportion of refusals, the long follow-up period, the short interval between follow-up visits (median, 6 months), the comprehensive information collected at baseline and during follow-up regarding risk factors, and the use of sensitive and well-validated PCR assays for the detection of HPV DNA in a central laboratory. By considering the time-dependent changing HR-HPV status in the analysis strategy, we have attempted to overcome our main limitation, which was the low number of CIN2/CIN3+ cases. This strategy maximizes the statistical power of the low number of cases observed in our study during follow-up.
Our results may not be directly applicable to clinical practice because the PCR methods used for the HR-HPV diagnosis are not the same as the Hybrid Capture II currently employed in clinical practice. Furthermore, colposcopy referral was performed based only on Pap smear results, which might lead to an under diagnosis of CIN2+. However, regarding the PCR test differences, there is evidence supporting that the sensitivity and specificity do not largely differ between the above mentioned two techniques.33 On the other hand, the potential under diagnosis of CIN2+ in our study could be similar to that observed in the vast majority of screening programs in which women are referred to colposcopy based on Pap smear results. Therefore, although our results are not comparable to those derived from trials or cohorts in which referral to colposcopy and biopsy is based on the results of the HPV test, they may closely reflect actual clinical practice scenarios, making our results more applicable, especially in Latin American countries.
In conclusion, the short-term absolute risk of CIN2/3 after a normal Pap smear with concurrent HR-HPV infection is low (~1%), with no observed differences according to age. In contrast to the strong negative predictive value of HPV testing, clinicians should understand the limited usefulness of an HR-HPV-positive result to short-term clinical decision-making for women with normal Pap smears.
Acknowledgments
Grant support was received from the Colombian Science & Research Department (COLCIENCIAS); the Department of Pathology, Vrije Universiteit Medical Center, the Netherlands; and from contract (#V501) between Merck Research Laboratories and the Instituto Nacional de Cancerologí-a, Colombia. We would like to thank Dr. Raul Murillo at INC, Colombia for his comments on the manuscript. We would also like to thank Dr. Alejandra Castanon at Wolfson Institute of Preventive Medicine, London UK for her comments and English proof-reading before submitting this manuscript for publication. The INC HPV study group: Drs. Monica Molano, Hector Posso, Margarita Ronderos, Mauricio González, Joaquí-n Luna, Gilberto Martí-nez, Edmundo Mora, Gonzalo Pérez, José Maria Fuentes, Constanza Gómez, Eva Klaus, Constanza Camargo, Cecilia Tobón, Teodolinda Palacio, Carolina Suárez, and Claudia Molina.
Declaration of conflicts of interest
We declare that we have no conflicts of interest.
References
1. Castellsague X, Munoz N. Chapter 3: Cofactors in human papillomavirus carcinogenesis--role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr 2003(31):20-8. [ Links ]
2. Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189(1):12-9. [ Links ]
3. Arbyn M, Paraskevaidis E, Martin-Hirsch P, Prendiville W, Dillner J. Clinical utility of HPV-DNA detection: triage of minor cervical lesions, follow-up of women treated for high-grade CIN: an update of pooled evidence. Gynecol Oncol 2005;99(3 Suppl 1):S7-11. [ Links ]
4. Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006;119(5):1095-101. [ Links ]
5. Wright TC, Jr., Schiffman M, Solomon D, et al. Interim Guidance for the Use of Human Papillomavirus DNA Testing as an Adjunct to Cervical Cytology for Screening. Obstet Gynecol 2004;103(2):304-9. [ Links ]
6. Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet 2003;362(9399):1871-6. [ Links ]
7. R. A. Smith, V. Cokkinides, and H. J. Eyre. American Cancer Society guidelines for the early detection of cancer, 2003. CA Cancer J Clin, January 1, 2003; 53(1): 27 - 43 (Accessed Sept 1, 2006, at http://caonline.amcancersoc.org/cgi/content/full/52/6/342. [ Links ])
8. Kitchener HC, Almonte M, Gilham C, et al. ARTISTIC: a randomised trial of human papillomavirus (HPV) testing in primary cervical screening. Health Technol Assess 2009;13(51):1-150, iii-iv. [ Links ]
9. Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007;370(9601):1764-72. [ Links ]
10. Naucler P, Ryd W, Tornberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007;357(16):1589-97. [ Links ]
11. Leinonen M, Nieminen P, Kotaniemi-Talonen L, et al. Age-specific evaluation of primary human papillomavirus screening vs conventional cytology in a randomized setting. J Natl Cancer Inst 2009;101(23):1612-23. [ Links ]
12. Lazcano-Ponce E, Lorincz AT, Salmeron J, et al. A pilot study of HPV DNA and cytology testing in 50,159 women in the routine Mexican Social Security Program. Cancer Causes Control, 2010. [ Links ]
13. Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer 2002;95(10):2145-51. [ Links ]
14. Sherman ME, Lorincz AT, Scott DR, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: a 10-year cohort analysis. J Natl Cancer Inst 2003;95(1):46-52. [ Links ]
15. Kjaer S, Hogdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res 2006;66(21):10630-6. [ Links ]
16. Carozzi F, Ronco G, Confortini M, et al. Prediction of high-grade cervical intraepithelial neoplasia in cytologically normal women by human papillomavirus testing. Br J Cancer 2000;83(11):1462-7. [ Links ]
17. Liaw KL, Glass AG, Manos MM, et al. Detection of human papillomavirus DNA in cytologically normal women and subsequent cervical squamous intraepithelial lesions. J Natl Cancer Inst 1999;91(11):954-60. [ Links ]
18. Sackett DL, Deeks JJ, Altman DG. Down with odds ratios! Evidence-Based Med 1996; 1: 164-166. [ Links ]
19. Molano M, Posso H, Weiderpass E, et al. Prevalence and determinants of HPV infection among Colombian women with normal cytology. Br J Cancer 2002;87(3):324-33. [ Links ]
20. Jacobs MV, Snijders PJ, van den Brule AJ, Helmerhorst TJ, Meijer CJ, Walboomers JM. A general primer GP5+/GP6(+)-mediated PCR-enzyme immunoassay method for rapid detection of 14 high-risk and 6 low-risk human papillomavirus genotypes in cervical scrapings. J Clin Microbiol 1997;35(3):791-5. [ Links ]
21. Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003;348(6):518-27. [ Links ]
22. Luff RD. The Bethesda System for reporting cervical/vaginal cytologic diagnoses: report of the 1991 Bethesda workshop. The Bethesda System Editorial Committee. Hum Pathol 1992;23(7):719-21. [ Links ]
23. Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol 2006;108(2):264-72. [ Links ]
24. Schlecht NF, Platt RW, Negassa A, et al. Modeling the time dependence of the association between human papillomavirus infection and cervical cancer precursor lesions. Am J Epidemiol 2003;158(9):878-86. [ Links ]
25. Hedeker D, Siddiqui O, Hu FB. Random-effects regression analysis of correlated grouped-time survival data. Statistical Methods in Medical Research 2000;9(2):161-79. [ Links ]
26. Munoz N, Mendez F, Posso H, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis 2004;190(12):2077-87. [ Links ]
27. Munoz N, Hernandez-Suarez G, Mendez F, et al. Persistence of HPV infection and risk of high-grade cervical intraepithelial neoplasia in a cohort of Colombian women. Br J Cancer 2009;100(7):1184-90. [ Links ]
28. Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Five-year experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol 2009;113(3):595-600. [ Links ]
29. Ronco G, Giorgi-Rossi P, Carozzi F, et al. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst 2008;100(7):492-501. [ Links ]
30. Castle PE, Rodriguez AC, Burk RD, et al. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. Bmj 2009;339:b2569. [ Links ]
31. Castle PE, Solomon D, Schiffman M, Wheeler CM. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J Natl Cancer Inst 2005;97(14):1066-71. [ Links ]
32. Cervical carcinoma and reproductive factors: collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int J Cancer 2006;119(5):1108-24. [ Links ]
33. Bozzetti M, Nonnenmacher B, Mielzinska II, et al. Comparison between hybrid capture II and polymerase chain reaction results among women at low risk for cervical cancer. Ann Epidemiol 2000;10(7):466. [ Links ]
Address reprints requests to: Dr. Gustavo Hernández-Suárez. Instituto Nacional de Cancerologí-a, Grupo de Investigación epidemiológica.
Av 1 No 9-85, tercer piso. Bogotá, Colombia.
E-mail: gahernandez@cancer.gov.co
Received on: May 10, 2010
Accepted on: September 29, 2010














