Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Salud Pública de México
versión impresa ISSN 0036-3634
Salud pública Méx vol.52 no.3 Cuernavaca may./jun. 2010
ARTICULO ORIGINAL
Adjunctive micronutrient supplementation for pulmonary tuberculosis
Suplementación con micronutrientes como tratamiento adjunto para tuberculosis pulmonar
Rodrigo X Armijos, MD, ScD,I; M Margaret Weigel, PhD,I; Rocío Chacon, MD,I; Luis Flores, RN, DPH,I, II; Armando Campos, MD.II
ICollege of Health Sciences, University of Texas at El Paso, USA
IIInstituto Mexicano del Seguro Social. Cd. Juárez, Mexico
ABSTRACT
OBJECTIVE: To assess the effect of micronutrient supplementation on tuberculosis (TB) patient outcomes.
MATERIAL AND METHODS: The randomized, double-blinded, placebo-controlled study was conducted in pulmonary TB patients undergoing directly observed treatment short course/ tratamiento acortado estrictamente supervisado (TAES/ DOTS) at IMSS in Ciudad Juarez, Chihuahua, Mexico, who were recruited during August 2005-July 2006. Consecutive patients received zinc and vitamin A supplements or matched placebo for four months. Dietary intake, blood zinc and vitamin A, immune response (IFN-γ,TNF-α, and IL-10 mRNA), and sputum smear conversion were measured.
RESULTS: The proportion of micronutrient compared to placebo group subjects with a negative sputum smear by month 3 was significantly increased (p= 0.03). This occurred subsequent to increased TNF-α and IFN-γ and decreased IL-'0 observed at month 2. Micronutrient supplementation appeared to accelerate the beneficial therapeutic effect of chemotherapy.
CONCLUSIONS: The earlier elimination of bacilli from sputum was associated with improved zinc status and Th' immune response. The therapeutic effect of vitamin A was less evident.
Key words: pulmonary; tuberculosis; zinc; vitamin A; cytokines; Mexico
RESUMEN
OBJETIVO: Determinar el efecto de la suplementación con zinc y vitamina A o placebo en pacientes tratados por tuberculosis (TB).
MATERIAL Y MÉTODOS: Se realizó un ensayo aleatorizado en pacientes tuberculosos que iniciaron el tratamiento acortado estrictamente supervisado/ directly observed treatment short course (TAES/DOTS) en las clínicas del IMSS, Ciudad Juárez, Chihuahua, México, reclutados durante agosto 2005-julio 2006. A cada paciente en forma aleatoria se le designó un código para recibir ya sea micronutrientes o placebo por cuatro meses, bajo el diseño doble ciego. Se evaluó la ingesta dietética, niveles de zinc y vitamina A en sangre, respuesta inmune (IFN-γ,TNF-α, IL-l0 mRNA en sangre) y bacilo ácido alcohol resistente (BAAR) en esputo.
RESULTADOS: Al tercer mes de la suplementación, la proporción de sujetos con BAAR negativo en el grupo de micronutrientes aumentó significativamente en relación con el grupo placebo (p= 0.03), que va asociado al previo (segundo mes) incremento de los niveles de TNF-α, e IFN-γ y disminución de los niveles de IL-10.
CONCLUSIONES: Suplementación con los micronutrientes aparentemente aceleran el efecto terapéutico de la quimioterapia. La negativización temprana del BAAR en esputo se asoció con la recuperación del estatus de zinc y la respuesta Thl. El efecto terapéutico de vitamina A es menos evidente.
Palabras claves: tuberculosis; pulmonar; zinc; vitamina A; citokinas; México
Vitamin A and zinc deficiencies are common features of pulmonary tuberculosis (TB).1-3 Deficiencies of both micronutrients can reduce host defenses and immune response.3,4 This can potentially affect host response to anti-TB chemotherapy and patient outcome. Regulatory T cells (Treg) and Th2 type immune response appear to predominate in the early clinical evolution of TB and becomes more pronounced as the disease worsens.5-7 However, successful chemotherapy causes a return back towards a Th1 state. Thus, improving the micronutrient status of treated pulmonary TB patients may accelerate bacterial clearance and clinical healing ostensibly through improvement of immune response.8
The evidence from prior studies is inconsistent regarding the potential therapeutic effects of vitamin A or zinc supplementation given alone or combined with other micronutrients.9-11 In addition, the putative effects of adjunctive vitamin A and zinc supplementation on the immune response TB patients has not been investigated. The randomized, placebo-controlled, double-blind pilot study was conducted to assess the effectiveness of adjunctive vitamin A and zinc supplementation on the nutritional status, immune response, and sputum smear conversion of patients being treated for pulmonary TB. It was hypothesized that supplementation would accelerate sputum smear conversion by improving Th1 immune response leading to macrophage activation and Mycobacteria killing.
Material and Methods
Newly diagnosed pulmonary TB patients attending the Instituto Mexicano del Seguro Social (IMSS) outpatient services in Ciudad Juárez, Chihuahua State, Mexico, were recruited between August 2005-July 2006. Consecutive patients with a positive sputum smear, without prior history of TB or treatment, and who were aged 18-65 years were eligible for participation unless they were pregnant, breastfeeding, used corticosteroids, or had HIV, diabetes, or another serious co-morbidity. All subjects gave their written informed consent. The protocol was approved by the IMSS, Universidad Autonoma de Ciudad Juárez, and University of Texas at El Paso institutional review boards.
Subjects were randomized to the micronutrient or placebo groups. Micronutrient group subjects received four months of supplementation with 5000 IU/ day of vitamin A as retinyl acetate and 50 mg/day elemental zinc as zinc chelate; placebo group subjects received organoleptically identical, matched placebos. A co-investigator not involved in data collection or analysis maintained the study codes and allocated the supplements. Subject codes remained sealed until after completion of data analysis. All subjects received short-course, directly observed antibiotic therapy per IMSS guidelines: intensive 60-day treatment with isoniazid (300 mg/ day), rifampicin (600 mg/day), pyrazinamide (1,600 mg/day) and ethambutol (1200 mg/day) followed by a sustained 45-dose therapeutic phase with isonizid (800 mg/dose) and rifampicin (600 mg/dose). Compliance with antibiotic and nutritional therapy was assessed on site at IMSS and during unscheduled home visits.
Face-to-face interviews and medical records were used to collect data on subject sociodemographic and clinical characteristics. Repeated 24-hour recalls, conducted at baseline and study months 1-4 and 6, estimated subject intakes of energy, zinc, vitamin A and other nutrients from food, supplements and other sources. The data were analyzed with the Food Processor diet analysis software. Subject BMI was calculated from observed weight and height data at baseline and months 1-4 and 6.
Ten mL blood samples were collected between 8-10:00 am for the nutritional and immunological analyses at baseline and months 2 and 6. Plasma zinc (inductively coupled plasma/optical emission spectroscopy) and serum retinol (HPLC) were analyzed. IFN-γ, TNF-α, and IL-10 mRNA cytokine analyses were performed at the UTEP Human Immunology and Nutrition Research Laboratory using quantitative RT-PCR (Applied Biosystems). Direct microscopy conducted at the IMSS clinical laboratory detected Mycobacteria present in sputum smears collected at baseline and months 1-6. Sputum cultures were analyzed for Mycobacteria complex/species (PCR) and antibiotic sensitivity (Middlebrook 7H11 + OADC) at the El Paso City-County Health Department Tillman Laboratory.
Subject baseline data were analyzed to verify random assignment of subjects. The intent-to-treat principal was used to examine treatment effect. Mantel-Haenzel X2 or Fisher's exact test analyzed differences between proportions and Students' independent and paired t-test for mean between- and within-group differences. The multivariate analyses employed ANCOVA.
Results
Thirty-nine subjects with confirmed pulmonary TB and antibiotic drug sensitivity were randomized to the micronutrient (n=20) or placebo groups (n=19). Of these, two subjects in the micronutrient group were lost to follow-up due to moving out of the city and one for non-compliance. One placebo group subject died and two others were lost either due to non-compliance or pregnancy. As Tables I-II indicate, no statistically significant between-group differences were identified in the baseline characteristics of the remaining subjects in the micronutrient (n=17) and placebo (n=16) groups.


Table II reveals that the mean dietary zinc intakes of the micronutrient but not placebo group showed
significant increases over baseline during all four supplementation months. The micronutrient group also had significantly higher mean zinc intakes compared to the placebo group during study months 1-4. However, significant between-group differences in mean vitamin A intake were identified only during months 2-3. A review of the 24-hour dietary data suggested that large intra-subject variations in the consumption of vitamin A-rich food sources were responsible (e.g., beef, liver, eggs). The two groups had comparable baseline and monthly mean intakes of energy, protein (Table II) and vitamin D, cholesterol and other major nutrients (data not shown) .
The micronutrient but not the placebo group showed a significant rise in mean plasma zinc levels at study month 2 compared to baseline (Table II). At month 2, the mean plasma zinc of the micronutrient group also was significantly higher compared to the placebo group. Although mean serum retinol levels steadily increased over baseline value as the study progressed, no significant between-group differences were recorded. This was most likely due to the previously noted subject intra-variation in vitamin A foods.
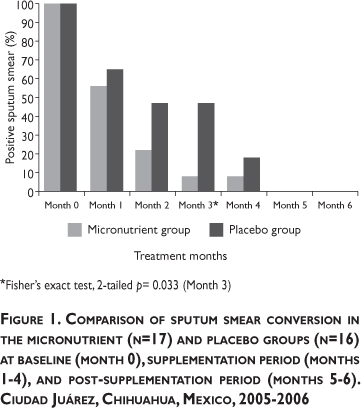
Figure 1 displays the proportion of subjects in the two study groups with a positive sputum smear from baseline through study month 6. The proportion of positive smears in both groups decreased over time until by the fifth month, none remained positive. The rate of decline was more pronounced in the micronutrient group but these differences only became statistically significant at month 3.
As Figure 2 shows, both subject groups exhibited a decreased Th1 immune response (i.e., low mRNA IFN-γ) and an elevated T regulatory (Treg) cytokine (mRNA IL-10) profile at baseline characteristic of untreated pulmonary TB.7,12 Increases in IL-10 and suppression of Th1 response have an adverse effect on IFN-γ availability. In addition, low TNF-α levels such as those seen at baseline may result from inadequate macrophage response. Progression of untreated Mycobacterium infection results in impairments in both innate and adaptive immune response leading to bacterial replication and disease chronicity (Figure 2).

Discussion
Similar to other reports,7 with chemotherapy, subject cytokine profiles began to shift more towards a Th1 immune response as indicated by increasing mRNA IFN-γ. The increasing mRNA TNF-α levels were also notable. The status of those two cytokines correlated with decreasing Treg cells activity (i.e., lower mRNA IL-10) in the present study. Although the between-group differences did not achieve statistical significance in this small pilot study, nonetheless, the observed trends in cytokine activity suggest that adjunctive micronutrient therapy acted to enhance bacterial elimination in the supplemented group (Figure 1).
The elevated IFN-γ and TNF-α mRNA levels identified for the micronutrient group at study month 2 is consistent with the observed acceleration in their sputum smear conversion rate signaling increased bacterial clearance. Although the TNF-α mRNA of the placebo group also increased over time, it did so more slowly, consistent with the longer time required for conversion to a negative smear. The post hoc analyses identified a significant positive correlation between plasma zinc and TNF-α mRNA levels at month 2 (r =0.47, p=0.03). The available evidence suggests that standard antibiotic TB treatment is associated with the upregulation of Th1 response, bacterial clearance and clinical improve-ment.13 In our study, adjunctive zinc supplementation appeared to accelerate this process. The effect of vitamin A supplementation was less evident.
The results of this initial study suggest that adjunctive micronutrient supplementation accelerated the beneficial therapeutic effect of TB chemotherapy by improving zinc status and Th1 immune response. Larger clinical studies are required to verify these initial results. If confirmed, adjunctive therapy could be used to shorten the amount of time that TB patients are contagious, thereby reducing the potential for disease spread and allowing them a faster return to work and society.
Acknowledgements
This project was supported by the Center for Border Health Research, an initiative of the Paso del Norte Health Foundation and the UTEP-UTSPH Hispanic Health Disparities Research Center. The authors also gratefully acknowledge the excellent technical assistance of Dr. Enrique Bravo, Dr. Carlos Porras, Dr. Julia Alvarez, Dr. Guadalupe Romero, Dr. Juana Trejo, Dr. Adriana Dominquez, Dr. Alberto Martinez, Febe Huitron, Genoveva Cordero, Marcela Gonzalez and Alma Lorena Rodriguez.
References
1. Karyadi E, Schultink W, Nelwan RHH, Gross R, Amin Z, Dolmans WMV, et al. Poor Micronutrient Status of Active Pulmonary Tuberculosis Patients in Indonesia. J Nutr 2002;130:2953-2958. [ Links ]
2. van Lettow M,West CE, van der Meer JW,Wieringa FT, Semba RD. Low plasma selenium concentrations, high plasma human immunodeficiency virus load and high interleukin-6 concentrations are risk factors associated with anemia in adults presenting with pulmonary tuberculosis in Zomba district, Malawi. Eur J Clin Nutr 2005; 59(4):526-532. [ Links ]
3. McMurray DN, Bartow RA, Mintzer CL, Hernandez-Frontera E. Micronutrient status and immune function in tuberculosis. Ann N Y Acad Sci 1990;587:59-69. [ Links ]
4. Karyadi E,West CE, Schultink W, Nelwan WH, Gross R, Amin Z, et al. A double-blind, placebo-controlled study of vitamin A and zinc supplementation in persons with tuberculosis in Indonesia: effects on clinical response and nutritional status. Am J Clin Nutr 2002;75(4):720-727. [ Links ]
5. Flynn J,Chan J. Immunology of tuberculosis. Ann Rev Immunol 200';19:93-129. [ Links ]
6. Boussiotis VA, Tsai EY,Yumis EJ, Thim S, Delgado JC, Dascher CC, et al. IL-10 producing T cells suppress immune response in anergic tuberculosis patients. J Clin Invest 2000;105:1317-1325. [ Links ]
7. Geffner L,Yokobori N, Basile J, Schierloh P, Balboa L, Romero M, et al. Patients with multidrug tuberculosis display impaired Thl responses and enhanced regulatory T-cell levels in response to an outbreak of multidrugresistant Mycobacterium tuberculosis M and Ra strains. Infection and Immunity 2009;77:5025-5034. [ Links ]
8. Karyadi E, Dolmans WM, West CE,Van Crevel R, Nelwan RH, Amin Z, et al. Cytokines related to nutritional status in patients with untreated pulmonary tuberculosis in Indonesia. Asia Pac J Clin Nutr 2007;16(2):218-226. [ Links ]
9. Range N,Andersen AB, Magnussen P, Mugomela A, Friis H. The effect of micronutrient supplementation on treatment outcome in patients with pulmonary tuberculosis: a randomized controlled trial in Mwanza, Tanzania. Trop Med Int Health 2005;10(9):826-32. [ Links ]
10. Chandra RK. Nutrient supplementation as adjunct therapy in pulmonary tuberculosis. Int J Vitam Nutr Res 2004;74(2):144-146. [ Links ]
11. Mathur M. Role of vitamin A supplementation in the treatment of tuberculosis. Nat Med J India 2007;20: 16-21. [ Links ]
12. Hernandez-Pando R, Orosco H, Aguilar D. Factors that deregulate the protective immune response in tuberculosis. Arch Immunol Ther Exp 2009;57:355-367. [ Links ]
13. Hirsch C, Toossi Z, Othieno C, Johnson J, Schwander S, Robertson S et al. Depressed T-Cell Interferon-g Responses in Pulmonary Tuberculosis: Analysis of Underlying Mechanisms and Modulation with Therapy. J Infect Dis 1999;180:2069-2073. [ Links ]
 Address reprint requests to:
Address reprint requests to:
Dr. RX Armijos
Human Immunology and Nutrition Research Laboratory
Department of Health Promotion/MPH Program
College of Health Sciences
University of Texas at El Paso
Stanton Professional Building Suite 700
1100 North Stanton Street. 79902-058
El Paso, Texas, USA
E-mail: rxarmijos@utep.edu
Received on: May 7, 2009
Accepted on: February 16, 2010














