Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Salud Pública de México
versión impresa ISSN 0036-3634
Salud pública Méx vol.51 supl.1 Cuernavaca ene. 2009
ARTÍCULO ORIGINAL
High dietary calcium intake decreases bone mobilization during pregnancy in humans
Alto consumo de calcio en la dieta, disminuye la movilización ósea durante el embarazo en seres humanos
Diana Avendaño-Badillo, MD, MCI; Mauricio Hernández-Ávila, MD ScDII, III; Leticia Hernández-Cadena, ScDIII; Gabriela Rueda-Hernández, MDIII; Maritsa Solano-González, BScIII; Luis G Ibarra, MDI; Howard Hu, ScD, MDIV; Martha M. Téllez-Rojo, ScDIII
INational Institute of Rehabilitation, Mexico
IIMinistry of Health, Mexico
IIINational Institute of Public Health, Mexico
IVDepartment of Environmental Health Sciences, University of Michigan. Ann Arbor, MI, USA
ABSTRACT
Calcium metabolism of the mother is modified during pregnancy because of the mineralization of the fetus skeleton.
OBJECTIVE: To evaluate the association of calcium intake and bone demineralization during pregnancy.
MATERIAL AND METHODS: At each trimester of pregnancy a validated food frequency intake questionnaire was administered to assess individual daily calcium intake in a cohort of 206 pregnant women, residents of Mexico City. Samples of urine were collected to measure levels of the cross-linked N-telopeptide of type I collagen (NTx), which is a biomarker of bone resorption. The association between calcium ingestion and bone resorption was analyzed using random effects models; non-linear associations were explored using generalized additive models.
RESULTS: Progressive increases in NTx levels were observed during pregnancy; with mean and standard deviation (SD) values during the first, second and third trimester of 76.50 (SD=38), 101.02 (SD=48.86) and 144.83 (SD=61.33) nmol BCE/mmol creatinine, respectively. Higher dietary calcium intake was associated with lower bone resorption (β=-0.015; p<0.05). The association between age and NTx showed a non-linear trend with an inflexion point around 33 years: increase in maternal age below that point was associated with a decrease in bone resorption, while in older women the increase in age was associated with an increased resorption.
CONCLUSIONS: Our results suggest that calcium ingestion, specifically from dairy products, reduces bone resorption during pregnancy. For each 300mg (a glass of milk) of calcium intake there is an estimated reduction in NTx level of 4.8 nmol BCE/mmol of creatinine (p<0.05).
Key words: pregnancy; bone resorption; N-telopeptides; calcium intake; longitudinal study; dairy products
RESUMEN
El metabolismo de calcio es modificado durante el embarazo debido a la mineralización del esqueleto del feto.
OBJETIVO: Evaluar la asociación entre la ingesta de calcio y la desmineralización ósea durante el embarazo.
MATERIAL Y MÉTODOS: Se administró un Cuestionario de Frecuencia de Consumo de alimentos en cada trimestre del embarazo para evaluar el consumo de calcio en una cohorte de 206 mujeres residentes de la Ciudad de México. Se recolectaron muestras de orina para medir los niveles de N-telopéptido de colágeno tipo I (NTx), biomarcador de resorción. Se hicieron modelos de efectos aleatorios; se estudiaron asociaciones no lineales utilizando modelos aditivos generalizados.
RESULTADOS: Se observó aumento progresivo en los niveles de NTx durante el embarazo. El mayor consumo de calcio se asoció con una menor resorción ósea (β=- 0.015, p<0,05).
CONCLUSIONES: Los resultados sugieren que la ingestión de calcio reduce la resorción ósea en el embarazo.
Palabras clave: embarazo; resorción ósea; N-telopéptidos; ingesta de calcio; estudio longitudinal; productos lácteos
During pregnancy, maternal calcium physiology adapts to meet the calcium demands of the growing fetus. Approximately 13 to 33 grams of calcium are needed for fetal ossification.1 Physiological responses to the demand for calcium during pregnancy include increased calcium absorption, renal calcium conservation and an increase in bone turnover during the third trimester.2-5 These changes occur as a result of modified levels of 1,25-dihydroxyvitamin D concentration, insulin-like growth factor (ilGF-I) and parathyroid hormone (PTH).6-8
Increases in bone mineral density (BMD) at cortical bone sites and decreases in BMD at trabecular bone sites3,4 have been associated with pregnancy and the latter suggests an increased risk for osteoporosis in subsequent years of life. However, studies conducted predominantly among populations with high dietary calcium intake indicate that neither extended lactation nor multiple pregnancies are associated with subsequent osteoporosis, whether measured by BMD levels or by assessment of fracture risk.9,10 It is possible that populations with an adequate calcium intake are able to compensate for bone mass lost during pregnancy and lactation.
There have been few studies aimed to understand calcium physiology during pregnancy that have been conducted among populations with low dietary calcium intakes.5,11,12 One study on a Mexican population of women with low calcium intakes (average calcium intake for pregnant women in Mexico has been estimated by the 1999 National Nutrition Survey at 565-800 mg/day) suggested that parity and lactation were inversely associated with BMD measured at later stages in life.13 However, these findings were not replicated in a recent study.14
A study conducted among women with low calcium intakes in Brazil5 concluded that calcium needs during pregnancy are partly met by high efficiency calcium absorption and renal calcium conservation, suggesting an important role for maternal dietary calcium intake. However, in that study the association between dietary calcium intake and bone remodeling was not explored. This is important because all studies conducted that have examined changes in bone density or biomarkers of bone remodeling suggest that bone remodeling increases during late pregnancy, concomitant with fetal ossification. This implies an important role of resorption and formation bone in contributing to the need of calcium at this stage of pregnancy.
In this study we longitudinally evaluated the changes in bone turnover during gestation to examine the association between dietary calcium intake and markers of bone turnover over the course of pregnancy. We evaluated the hypothesis that dietary calcium intake will be inversely associated with markers of bone turnover.
Material and Methods
All participants were healthy women between 15 and 43 years of age recruited between May 1997 and April 1999 from one of four prenatal care clinics of the Mexican Institute of Social Security (IMSS, per its abbreviation in Spanish) in Mexico City to participate in a study to assess the relation between different lead biomarkers over the course of pregnancy and lactation.15 Women were eligible for participation if they were willing to declare intention to remain available throughout the study and desired to become pregnant in the near future or had not yet reached gestational week 14 in a current pregnancy. Women were excluded from the study if they did not intend to breastfeed, had illnesses that modified their calcium metabolism, received a physician's diagnosis of multiple pregnancies, or had other medical problems including history of preeclampsia or pregnancy-related hypertension, psychiatric or cardiac disease, gestational diabetes, history of frequent urinary tract infections, seizure disorders requiring daily medications or ailments that required medication with corticosteroids.
Participants went to the Mexican National Institute of Public Health (INSP) Research Facility in Mexico City for a baseline evaluation that included environmental risk factor assessment, a food frequency questionnaire to evaluate dietary calcium intake, and a physical exam including a urine sample to measure N-telopeptide (NTx) of type I collagen –a biomarker of bone resorption. Women were scheduled for follow-up evaluations at 12, 24, and 34 weeks of gestational age and at 1, 3, 7, and 12 months postpartum. Questionnaire data, maternal anthropometry, and samples of urine (second morning sample), blood and plasma for lead analysis were collected during follow-up visits. Anthropometric measurements were conducted by trained nurses via standardized methods.
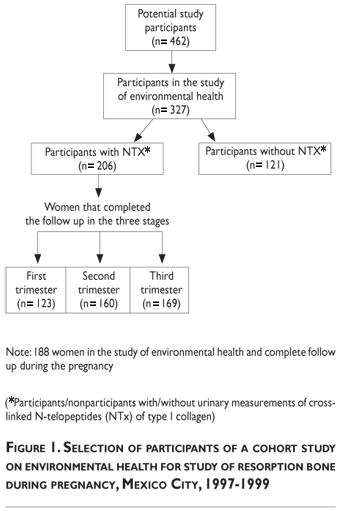
Collection of urine samples was implemented several months after the cohort was assembled, reducing the number of participants represented by NTx data to 206. This subgroup of 206 participants will be subsequently referred to as "participants in the NTx study" (Figure 1). Urine was collected and stored in clean containers which were frozen to -70°C and shipped to the Harvard School of Public Health Toxicology Laboratory for cross-linked N-telopeptides analysis. Samples were analyzed with a commercially available competitive-inhibition enzyme-linked immunosorbent assay (Osteomark; Ostex International; Seattle, WA). NTx concentrations were expressed as nanomoles of bone collagen equivalents normalized to creatinine (nmol BCE/mmol creatinine). The intra- and inter-assay coefficients of variation were below 10%. The analysis was blinded to characteristics of the participants other than urine NTx concentration.
Daily calcium and calorie intake was assessed in each trimester using a self-administered food frequency questionnaire (FFQ) of 82 foods, developed and validated among women living in Mexico City using a previously reported methodology.16 The FFQ listed foods that were predictive of nutrient ingestion to quantify the relative ingestion of various micronutrients. For each food listed, the amount was specified according to common portion sizes, such as a cup, an egg, or a tortilla, whenever possible. A specific computer program was developed to derive the relative ingestion of each specific nutrient.
To evaluate the association between calcium ingestion and urinary NTx concentration over the course of pregnancy, we generated two longitudinal multivariate linear models adjusted by participant age, height and gestational age. In a first approach, we evaluated the total ingestion of calcium. In a second approach, we divided the total calcium intake into either dairy or non-dairy sources. We adjusted for total caloric intake in both approaches. NTx concentration was log-e transformed because it was not normally distributed. To assess the robustness of our findings, we repeated analyses including women with complete information in the three trimesters (n=92). To assess the potential effect of a threshold, we explored the non-linear associations between all predictors and the outcome variable using generalized additive models (GAM).17 The correlation due to repeated measurements over time on the same subject was taken into account through a random intercept.18 The statistical analysis was conducted with Stata 7 and S-Plus 2000.
The research protocol used was approved by the Human Subjects Committees of the National Institute of Public Health of Mexico (INSP), the Harvard School of Public Health, and by the participating hospitals. All of the subjects received and signed a detailed written informed consent form, including an explanation of the study and its procedures.
Results
The mean age of the participants was 27 years (range 15–43 years) and there were no significant differences in age, height, weight, hemoglobin, hematocrit, calcium intake, parity, previous number of pregnancies, and education between women who participated in the NTx substudy and those who did not (Table I).
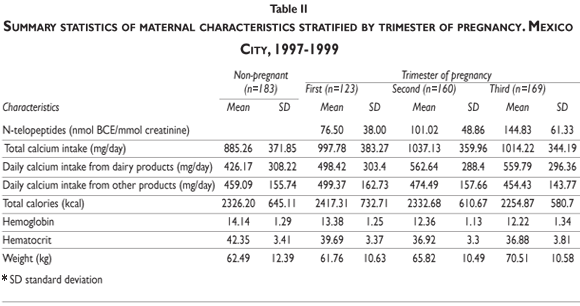
Table II displays summary statistics of NTx, dietary calcium ingestion (from dairy and non-dairy products), calories, hemoglobin, hematocrit, and maternal weight by trimester of pregnancy alongside corresponding statistics for the reference group of 183 non-pregnant women. Also shown are the descriptive statistics of maternal characteristics. The calcium ingestion from non-dairy sources was similar in both pregnant and non-pregnant women, although there is a slightly more dairy-based calcium intake in the pregnant women than in the non-pregnant (997.78 SD=383.27 versus 885.26, SD=371.85). NTx (SD) increased (p<0.01) over the course of pregnancy, with mean levels of 76.50 (38.00), 101.02 (48.86) and 144.83 (61.33) nmol BCE/mmol creatinine in the first, second, and third trimesters, respectively.
After using a longitudinal multivariate model to adjust for height, maternal age, gestational age,and caloric intake (Table III, model Total calcium), we detected a significant inverse association between calcium intake and NTx (log-e transformed). When we split total calcium intake into dairy and non-dairy products, an inverse association with NTx was detected for both sources, but only the former was statistically significant (Table III, model Intake Calcium). Also, we found a negative association between adjusted means for NTx and daily calcium intake from dairy products.
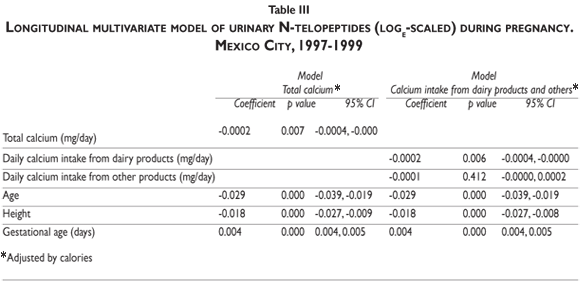
Figure 2 shows the linear and non-linear results obtained from an additive model. Calcium intake and maternal height showed a negative linear association with NTx, while a threshold was detected for gestational and maternal age. We estimated that every 300g of calcium intake was associated with a 4.8nM BCE/mM creatinine decrease in bone resorption marker, β=-0.016 (p<0.05). Regarding gestational age, our results suggest that there is an initial positive association until approximately 160 days of gestation, followed by a steeper positive association reflecting a more accelerated bone resorption activity in the second half of pregnancy. This trend was significantly different from a linear curve (p<0.01) and suggests the existence of a threshold. Additionally, a clear non-linear association between maternal age and NTx was detected. This association was negative until approximately 30 years of age. Between 30 and 34 years of age a threshold was detected and after this period there is a significant positive deviation (p<0.01).
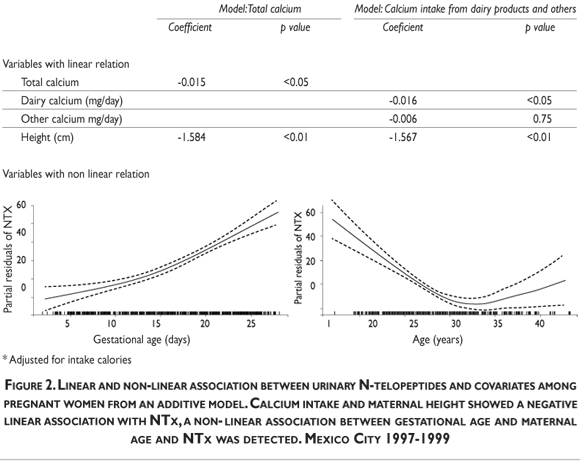
To assess the robustness of our findings, we repeated the analyses in the sub-sample of women who completed follow-up and had no missing data over the three trimesters of pregnancy (n=92). Since the results were essentially the same, only models which incorporated all the available information are presented.
Discussion
In this longitudinal study we observed a linear negative association between dietary calcium intake and biomarkers of bone resorption during pregnancy. We also documented that height and age were important determinants of bone resorption.
Our results show that NTx levels were greatest in the third trimester, which is consistent with other studies that have observed that ultrasonographic maternal bone propagation velocity decreases in the second and third trimesters19-23 and studies that have shown increases in various bone resorption biomarkers (DPyr, Pyr, CTx and NTx) principally during the third trimester.24-27 Our observation that the greatest increase in NTx values occurs after approximately 160 days of gestation may reflect an increase in the fetal demand for mineralization and therefore greater bone resorption in the second half of pregnancy.25,27,28
The significant negative correlation we found between NTx and dietary calcium intake suggests that dietary calcium does alter skeletal response to the increased demand for calcium during pregnancy and that increases in bone resorption during this period may be attenuated by parallel increases in dietary calcium. This is concordant with a study conducted in 23 adolescent mothers29 that documented significant decrements in bone density measured in the lumbar spine during pregnancy and observed that an increased calcium intake during pregnancy appeared to be protective against maternal loss of trabecular bone at the lumbar spine. Our results are also similar to those reported by Zeni et al11 who showed longitudinal changes of bone turnover during pregnancy among a group of 39 pregnant women and documented an increase in bone resorption markers, with the highest increment observed in the third trimester and a significant negative correlation between NTx and dietary calcium intake. Protective results of a 1200 mg calcium supplementation on bone resorption were also documented in a randomized crossover trial among 31 pregnant women in Mexico City.30 The benefits of calcium intake may not only reduce bone resorption during pregnancy but also may inhibit bone lead mobilization, an endogenous source or prenatal lead exposure 15.
In our study, the separation of calcium between dairy and non-dairy sources may have provided an improved estimate of calcium intake. Although a recent review of calcium bioavailability31 concluded that there is no evidence to suggest that the calcium in milk is more efficiently used than any calcium salt, it is known that the calcium in milk and dairy products is better absorbed than the calcium in spinach or watercress because the plants have high oxalate contents. Among the Mexican population in our study, tortillas are a staple of the diet and an important source of non-dairy dietary calcium. Calcium in tortillas is compromised by phytate, which is present in large quantities in maize kernels, thereby reducing its bioavailabity.
The reduced sample size of this study compromises the exploration of a mother's age as a potential modifier of bone remodeling activity during pregnancy. However, we observed that bone resorption activity was higher in younger women and that an inflection point was detected around 34 years of age, where older women showed an increasing trend toward bone resorption. There are no reported studies regarding the effect of maternal age to which we could compare our results; further research may help to clarify the biological importance of maternal age with regard to bone remodeling during pregnancy.
Another limitation to our study is that we did not measure other biomarkers of bone metabolism or hormonal changes and therefore were unable to develop a fully integrative view of bone metabolism during pregnancy. A previous study performed throughout the course of pregnancy on an Asian population reports a bone turnover ratio reflecting stronger bone resorption than bone formation when measured with osteocalcin.28 Another study on a Caucasian population found increases in bone formation during the second and third trimesters, 21% and 25% respectively, as measured by specific alkaline phosphate levels in bone (b-ALP), and 44% and 133%, respectively, with regard to carboxyterminal propeptide of procollagen type 1(PICP).11 The stage of pregnancy at which the imbalance between resorption and formation is highest is still a matter of controversy.11, 25, 28 Disagreements between study results may be explained by the fact that different biomarkers of bone formation reflect different aspects of the dynamics of bone formation and that different populations studied may have unequal levels of b-ALP or other biomarkers.32
Our results suggest the need to evaluate dietary requirements for calcium during pregnancy and the need to conduct additional studies to further explore the association between pregnancy and lactation and the risk of osteoporosis within populations with lower calcium intake that experience pregnancy at earlier ages. Additional information is also needed about the adolescent population that has not yet reached peak bone density mass and may be more vulnerable to the effects of pregnancy on bone health.
It is also important to conduct new studies to assess the influence of genetic factors and lifestyle on increased bone resorption and to simultaneously evaluate bone resorption and bone formation processes during a period of life that is characterized by an accelerated bone metabolism process. Investigations should be undertaken to further explore the possible effects of calcium supplementation and the use of NTx as a diagnostic tool for identifying women at increased risk for bone resorption. Some final recommendations would be to improve the milk-based calcium ingestion of pregnant women, especially among younger women.
Acknowledgments
We acknowledge the American British Cowdray Medical Center for providing us with the research facilities to conduct the study. This research was made possible by grants RO1 ES007821 and 5P42ES05947 from the US National Institute of Environmental Health Sciences (Research Triangle Park, North Carolina), by NCRR GCRC M01RR02635 from the US NIH, and by grants 29192-M and 38867-M from the Mexican National Council of Science and Technology (CONACyT) (Mexico City, Mexico). The contents of this article are solely the responsibility of the authors.
References
1. Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocrine Reviews 1997;18:832-872. [ Links ]
2. Heaney RP, Skillman TG: Calcium metabolism in normal human pregnancy. J Clin Endocr 1971;33:661-670. [ Links ]
3. Naylor KE, Iqbal P, Fledelius C, Fraser RB, Eastell R.The effect of pregnancy on bone density and bone turnover. J Bone Miner Res 2000;15:129-137. [ Links ]
4. Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, King JC. A longitudinal study of calcium homeostasis during pregnancy and lactation and after the resumption of menses. Am J Clin Nutr 1998;67:693-701. [ Links ]
5. Vargas Zapata CL, Donangelo CM, Woodhouse LR, Abrams SA, Spencer EM, King JC. Calcium homeostasis during pregnancy and lactation in Brazilian women with low calcium intakes: a longitudinal study. Am J Clin Nutr 2004;80:417-422. [ Links ]
6. Kovacs CS. Calcium and bone metabolism in pregnancy and lactation. J Clin Endocrin Metab 2001;86:2344-2348. [ Links ]
7. King JC. Effect of reproduction on the bioavailability of calcium, zinc and selenium. J Nutr 2001;131:1355S-1358S. [ Links ]
8. Prentice A.Maternal calcium metabolism and bone mineral status. Am J Clin Nutr 2000;71(suppl):1312S-1316S. [ Links ]
9. Karlsson C, Obrant KJ, Karlsson M. Pregnancy and lactation confer reversible bone loss in humans. Osteoporos Int 2001;12:828-834. [ Links ]
10. Paton LM, Alexander JL, Nowson CA, Margerison C, Frame MG, Kaymakci B, Wark JD. Pregnancy and lactation have no longterm deleterious effect on measures of bone mineral in healthy women: A twin study. Am J Clin Nutr 2003;77:707-714. [ Links ]
11. Zeni SN, Ortela Soler CR, Lazzari A, Lopez L, Suarez M, Di Gregorio S, Somoza JI, de Portela ML. Interassociation between bone turnover markers and dietary calcium intake in pregnant women: a longitudinal study. Bone 2003;33:606-613. [ Links ]
12. Prentice A, Jarjou LMA, Stirling DM, Buffenstein R, Fairweather-Tait S. Biochemical markers of calcium and bone metabolism during 18 months of lactation in Gambian women accustomed to a low calcium intake and in those consuming a calcium supplement. J Clin Endocrinol Metab 1998;83:1059-1066. [ Links ]
13. Parra-Cabrera S, Hernandez-Avila M, Tamayo-y-Orozco J, Lopez-Carrillo L, Meneses-González F. Exercise and reproductive factors as predictors of bone density among osteoporotic women in Mexico City. Calcif Tissue Int 1996;59:89-94. [ Links ]
14. Lopez-Caudana AE, Tellez-Rojo Solis MM, Hernandez-Avila M, Clark P, Juarez-Marquez SA, Lazcano-Ponce EC, Salmeron-Castro J. Predictors of bone mineral density in female workers in Morelos State, Mexico.Arch Med Res. 2004;35:172-180. [ Links ]
15. Tellez-Rojo MM. Hernandez-Avila M. Lamadrid-Figueroa H. Smith D. Hernandez-Cadena L. Mercado A. Aro A. Schwartz J. Hu H. Impact of bone lead and bone resorption on plasma and whole blood lead levels during pregnancy. Am J Epidemiol 2004;160:668-678. [ Links ]
16. Hernandez-Avila M. Romieu I. Parra S. Hernandez-Avila J. Madrigal H. Willett W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Pub Mex 1998;40:133-140. [ Links ]
17. Hastie TJ, Tibshirani RJ. Generalized additive models. London: Chapman and Hall, Inc. 1990. Methodology, biostats section. [ Links ]
18. Brown H, Prescott R. Applied Mixed Models in Medicine. Chichester: John Wiley and Sons; 2001. [ Links ]
19. Yamaga A, Taga M, Minaguchi H, Sato L. Changes in bone mass as determined by ultrasound and biochemical markers of bone turnover during pregnancy and puerperium: A longitudinal study. J Clin Endocrinol Metab 1996;81:752-756. [ Links ]
20. Kolthoff N, Eiken P, Kristensen B, Nielsen SP. Bone mineral changes during pregnancy and lactation: a longitudinal cohort study. Clin Sci 1998;94:405–412. [ Links ]
21. Aguado F, Revilla M, Hernandez ER, Menendez M, Cortes-Prieto J, Villa LF, Rico H. Ultrasonographic bone velocity in pregnancy: a longitudinal study. Am J Obstet Gynecol 1998;178:1016-1021. [ Links ]
22. To WK, Wong MWN, Leung TW, Association between bone mineral density changes in pregnancy and maternal and pregnancy characteristics: a longitudinal study. Acta Obstet Gynecol Scand 2003;82:820-827. [ Links ]
23. Gambacciani M. Spinetti A. Gallo R. Cappagli B. Teti GC. Facchini V. Ultrasonographic bone characteristics during normal pregnancy: longitudinal and cross-sectional evaluation. Am J Obs Gynecol 1995;173:890-893. [ Links ]
24. More C, Bhattoa HP, Bettembuk P, Balogh A. The effects of pregnancy and lactation on hormonal status and biochemical markers of bone turnover. European J Obstet Gynec Reprod Bio 2003;106:209-213. [ Links ]
25. Black, AJ, Topping J, Durham B, Farquharson RG, Fraser WD. A detailed assessment of alterations in bone turnover, calcium homeostasis, and bone density in normal pregnancy. J Bone Miner Res 2000;15:557-563. [ Links ]
26. Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and post-weaning: a longitudinal study. Am J Clin Nutr 1995;61:514-523. [ Links ]
27. Yamaga A, Taga M, Minaguchi H. Changes in urinary excretions of C-telopeptide and cross-linked N-telopeptide of type 1 collagen during pregnancy and puerperium. Endocrine J 1997;44:733-738. [ Links ]
28. Yoon B-K, Lee J-W, Choi D, Roh C-R, Lee J-H. Changes in biochemical bone markers during pregnancy and puerperium. J Korean Med Sci 2000;15:189-193. [ Links ]
29. O'Brien KO, Nathanson MS, Mancini J, Witter FR. Calcium absorption is significantly higher in adolescents during pregnancy than in the early postpartum period. Am J Clin Nutr 2003;78:1188-1193. [ Links ]
30. Janakiraman V, Ettinger A, Mercado-Garcia A, Hu H, Hernandez-Avila M. Calcium supplements and bone resorption in pregnancy. Am J Prev Med 2003;24:260-264. [ Links ]
31. Guéguen L, Pointillart A. The bioavailability of dietary calcium. J Am Coll Nutr 2000; 19:119S-136S. [ Links ]
32. Gundberg CM, Looker AC, Nieman D, Calvo MS. Patterns of ostecalcin and bone specific alkaline phosphatase by age, gender, and race or ethnicity. Bone 2002;31:703-708. [ Links ]
Received on: November 10, 2008
Accepted on: December 11, 2008
Address reprint requests to: Martha M. Téllez-Rojo. Oficina #325. Instituto Nacional de Salud Pública. Av. Universidad #655 Col Sta. Ma. Ahuacatitlán. Cuernavaca, Mor. México. CP 62508.
E-mail: mmtellez@insp.mx














