Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Salud Pública de México
versión impresa ISSN 0036-3634
Salud pública Méx vol.51 no.1 Cuernavaca ene./feb. 2009
ARTÍCULO ORIGINAL
Rapid response to a case of mumps: implications for preventing transmission at a medical research facility
Respuesta rápida a un caso de paperas: implicaciones para la prevención del contagio en una instalación de investigación biomédica
Gabriela Salmón-Mulanovich, BScI; Gregory Utz, MDI, II; Andrés G Lescano, MHS, PhDI; David E Bentzel, VDM, MPH, DACLAMI; David L Blazes, MD, MPHI
INaval Medical Research Center Detachment. Peru
IIInfectious Diseases / HIV Clinic, Naval Medical Center. San Diego, California
ABSTRACT
OBJECTIVE: To prevent transmission among the staff and potentially among the non-human primate (NHP) colony at the U.S. Naval Medical Research Center Detachment in Peru, where an active case of mumps was discovered in a senior laboratory technician in Sep 03, 2007.
MATERIAL AND METHODS: Subjects at the research facility were interviewed and potentially susceptible contacts were tested for mumps IgG.
RESULTS: In total, 81 out of 106 staff members (76%) had close contact with the case. Only 6/81 (7%) had MMR, 33 (41%) reported having had mumps, and 8 of 45 (18%) of the potentially susceptible individuals did not have immunity (IgG > 20.0). All the susceptible, exposed individuals received MMR vaccine. There were no secondary cases and access to the NHP colony was restricted.
DISCUSSION: Immediate and thorough investigation and occupational health response were imperative in preventing secondary cases of mumps among humans and NHP.
Key words: mumps; epidemiology; primates
RESUMEN
OBJETIVO: Prevenir el contagio de parotiditis al personal y potencialmente a la colonia de primates no humanos (PNH), tras detectarse un caso en el personal técnico de laboratorio en el Centro de Investigación de Enfermedades de la Marina de los EUA (NMRCD).
MATERIAL Y MÉTODOS: El personal fue entrevistado y se hizo una prueba de IgG para parotiditis a los contactos potencialmente susceptibles.
RESULTADOS: En total, 81 de 106 miembros del personal tuvo contacto cercano con el caso. Sólo 6/81 (7%) tenían vacuna y 33 (41%) reportaron haber tenido parotiditis, y 8 de 45 (18%) de los susceptibles potenciales no tenían inmunidad (IgG > 20.0). Todos los susceptibles expuestos fueron vacunados y no hubo casos secundarios. Se restringió el acceso a la colonia de PNH.
CONCLUSIÓN: La investigación inmediata y la respuesta de salud ocupacional fue imperativa para prevenir casos secundarios de parotiditis en el personal y los NHP.
Palabras clave: parotiditis; epidemiología; primates
Mumps is a disease caused by a paramyxovirus that is transmitted through direct contact with saliva droplets. Mumps became part of the mandatory childhood vaccination scheme in Peru in 2003 and is now provided as part of the measles-mumps-rubella vaccine, but there is likely a sizable proportion of the population that is non-immune, not unlike some localities in the United States where vaccination has been eschewed.
In September 2003, a case of mumps was discovered in a senior laboratory technician at the Naval Medical Research Center Detachment (NMRCD) in Lima, Peru. Infection control measures and an investigation were promptly conducted to minimize transmission to non-immune personnel and prevent infection of NMRCD's colony of non-human primates (NHP), Aotus nancymae. Although the natural host for this virus is the human, there are reports in the literature that non-human primates (marmosets) are susceptible to mumps.1 In 1934, Johnson showed that mumps could be transmitted from infected patients to rhesus monkeys.2 Likewise, rhesus monkeys experimentally infected with mumps virus display clinical signs similar to humans, including parotid gland enlargement and edema of the surrounding tissues, although usually in the absence of fever.2-5 This raised some concern that the NHP colony of Aotus nancymae might become affected. Hence, the objective of the study was to prevent transmission among the staff and potentially the non-human primate (NHP) colony at NMRCD.
Materials and methods
A retrospective cohort study was conducted. All the staff completed a questionnaire regarding their exposure to the index case through the entire contagious period. The period of transmission was calculated to be from September 15th-28th, from three days before symptoms appeared to approximately nine days after. Personnel that were identified as contacts of the index case were asked about their prior history of clinical mumps. Ten cc of whole blood was collected from the contacts that had not received prior vaccination against mumps or lacked a history of parotitis, after acquiring their verbal consent. The serum was tested for IgG using EIAgen Mumps IgG, Biochem Immunosystems, Italy. The cut-off point was set at titers > 20.0 U/mL.
The Naval Medical Research Center Institutional Review Board determined that the investigation (PJT-24) did not meet the definition of human subject research, since it was framed as an outbreak investigation. Nonetheless, staff provided their verbal consent before having blood drawn and answering the questionnaire.
Results
The index case was identified and confirmed by the occupational health physician at the facility. The case had right-sided parotitis and reported having fever and malaise the previous day (September 18th). She apparently acquired the disease from her son who was clinically diagnosed with mumps 14 days earlier (September 5th). To prevent secondary transmission, the technician was dismissed from work until September 29th. All the staff present at NMRCD (n=106) were asked if they had had contact with the case from September 15th to the day of the investigation, September 19th. Eighty-one staff members (77%) had contact with the index case during a fund raising breakfast on September 16th, including two pregnant women. The index case had served food during the breakfast. The timeline for these events is shown in figure 1.
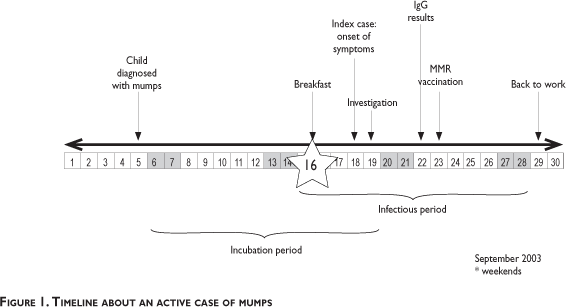
Among the contacts of the index case, 46 did not have a history of the disease or of receiving the vaccine (see figure 2). With the exception of one subject who was lost for follow-up, forty-five potentially susceptible staff members were tested for immunity via IgG antibody titers. Thirty-eight contacts had titers over 20.0 U/mL, confirming previous infection and immunity. This group with unknown pre-existing immunity included one pregnant subject; the other pregnant woman had a previous history of mumps. Thus, within the contact group, a total of 72/81 (89%) were probably immune and 8/81 (10%) naïve. The characteristics for the group of immunized individuals, personnel with a history of mumps and people of unknown immune status are shown in table I.
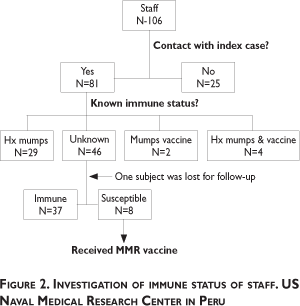
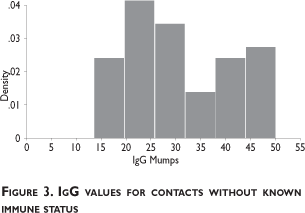
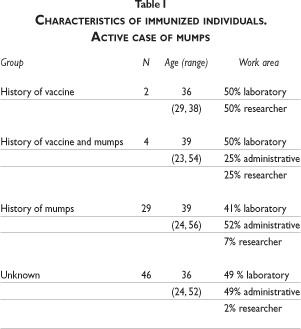
The naïve group was mostly women (5/8, 63%), and mostly from administrative positions (6/8, 75%); all were offered MMR vaccine on September 23rd, after receiving counseling from NMRCD's occupational health physician.
No secondary cases of mumps were observed in NMRCD personnel and the NHP colony of Aotus nancymae was not affected.
Discussion
The occurrence of this mumps case acquires more relevance because in the past few years, numerous outbreaks of mumps have been reported in the literature, even among supposedly well immunized populations.6,7 The reason for these outbreaks is likely multi-factorial, including waning immunity, vaccine failure and most importantly, decreasing vaccine coverage of susceptible populations. Although the benefits and safety of the MMR vaccine have been well documented, recently there has been a movement among certain segments of the lay public to refuse to vaccinate their children for fear of them developing autism.8-10 This supposition has been soundly refuted in the medical literature, but the belief persists among certain segments of the population and has led to at least one well documented outbreak in Iowa, USA.11
In the case at hand, the effect of the control measures cannot be definitively confirmed. The literature reports that approximately a third of all cases of mumps are asymptomatic.12 Therefore, the lack of secondary cases may be due to the interventions, lower transmission to secondary cases, subclinical infections that were undetected or a large cohort of personnel with high levels of pre-existing immunity. Applying the herd immunity concept to such a small population has limitations, but 88% of the exposed population (71/80) were found to be immune, which is similar to the calculated threshold for herd immunity for mumps ranging from 86-92%.12, 13 Additionally, the effectiveness of one dose of vaccine reported during outbreaks in other settings has been calculated to be between 65 and 87.8%. 7, 13, 14
The uncertainty surrounding the immune status of laboratory personnel generated much personal concern, missed work and expense. The value of worry is impossible to calculate, but as a rough estimate, the actual costs of having personnel on sick leave, of undertaking the investigation (occupational health personnel, testing, specimen handling, etc.) and of the control measures (vaccination) totaled US$ 2421. If the animal facility had been affected, the costs would have increased to US$ 142,429, just to replace the NHP colony, with additional costs of animal disposal and the priceless months of research lost. On the other hand, the cost of vaccinating every person at the laboratory when they were first hired would have been less than US$6 for each individual, for a total of US$ 624 for the whole 106 staff members. Finally, because this was not (and still is not) a reportable disease in Peru, it was not possible to elucidate the situation in the community.
The World Health Organization (WHO) recommends mumps immunization in countries with well established vaccination programs which can maintain a high level of vaccination coverage. In the research facility, vaccinating all the personnel (n=106) would have cost US$ 624 plus the expenses of application and follow up. This approach, although initially less expensive, was not chosen, as it would require close follow up of all the staff and continued state of alert and concern among the personnel. Additionally, it would not help determine if the laboratory animals were being exposed to further risk by allowing potentially infectious individuals in the facility, since immunity could not be ensured through immunization in a potential incubation period.
Very limited data are available regarding the impact of an outbreak of mumps in the developing world, let alone a research facility located there. It can be concluded that the case study presented herein uses this setting to underscore the potential hazards of decreasing vaccine coverage in the United States or other developed countries. In addition, mumps and other vaccine preventable diseases should be high priorities for populations where vaccine coverage is low and there is potential for outbreaks with significant morbidity among humans and possibly non-human primates.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government.
Source of support: This work was funded by DoD-GEIS and supported by work unit number 847705 82000 25GB B0016.
IRB statement: The Naval Medical Research Center Institutional Review Board determined the investigation (PJT-24) did not meet the definition of human subject research.
Copyright statement: The authors are military service members and employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that "Copyright protection under this title is not available for any work of the United States Government." Title 17 U.S.C. §101 defines U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties.
Conflict of interest: the authors declare no conflict of interest or significant financial interest to disclose/ related to the entity funding the project or any other institution involved with the investigation
Acknowledgements
The authors would like to thank the personnel from NMRCD who patiently participated in this investigation, especially the index case and her family.
References
1. Saika S, Kidokoro M, Ohkawa T, Aoki A, Suzuki, K. Pathogenicity of mumps virus in the marmoset. J Med Virol 2002; 66(1): 115-122. [ Links ]
2. Johnson CD, Goodpasture EW. Experimental immunity to the virus of mumps in monkeys. Am J Epidemiol 1936; 23(2):329-339. [ Links ]
3. Findlay GM, Clarke LP. Experimental production of mumps in monkeys. Brit J Exp Pathol 1934; 15:309-313. [ Links ]
4. Enders JF, Kane LW, Cohen S, Levens J. Immunity in mumps: I. Experiments with monkeys (Macacus mulatta). The development of complement-fixing antibody following infection and experiments on immunization by means of inactivated virus and convalescent human serum. J Exp Med 1945; 81(1): 93-117. [ Links ]
5. Flanagan TD, Andrada JA, Barron AL, Witebsky E. Response to experimental infection with mumps virus in rhesus monkeys. Infect Immun 1971; 3(5):642-647. [ Links ]
6. Center for Disease Control and Prevention. Mumps outbreak at a summer camp—New York, 2005. MMWR Morb Mortal Wkly Rep 2006; 55(7): 175-177. [ Links ]
7. Sartorius B, Penttinen P, Nilsson J, Johansen K, Jonsson K, Arneborn M, et al. An outbreak of mumps in Sweden, February-April 2004. Euro Surveill 2005; 10(9): 191-193. [ Links ]
8. Caplan CE. Mumps in the era of vaccines. CMAJ 1999; 160(6):865-866. [ Links ]
9. Wilson TR, Fishbein D B, Ellis P A, Edlavitch S A. The impact of a school entry law on adolescent immunization rates. J Adolesc Health 2005; 37(6):511-516. [ Links ]
10. Clements C, Ratzan S. Misled and confused? Telling the public about MMR vaccine safety. Measles, mumps, and rubella. J Med Ethics 2003; 29(1): 22-26. [ Links ]
11. Center for Disease Control and Prevention. Mumps epidemic—Iowa, 2006. MMWR Morb Mortal Wkly Rep 2006; 55(13): 366-368. [ Links ]
12. Anderson RM, Crombie JA, Grenfell BT. The epidemiology of mumps in the UK: a preliminary study of virus transmission, herd immunity and the potential impact of immunization. Epidemiol Infect 1987; 99(1): 65-84. [ Links ]
13. Cohen CWJ, Savage EJ, Glynn JR, Choi Y, Andrews N, et al. Vaccine effectiveness estimates, 2004-2005 mumps outbreak, England. Emerg Infect Dis 2007; 13(1). Available from http://www.cdc.gov/ncidod/EID/13/1/12.htm [ Links ]
14. Fernandez-de la Hoz-Zeitler K, Garcia-Colmenero C, Puchades-Belenguer M J, Verde-Lopez C, Carpintero-Redondo J L, Alcazar-Casanova F. Mumps epidemic in the health area of Toledo with immunization intervention. Estimation of vaccine effectiveness. Med Clin (Barc) 1997; 108(5): 175-179. [ Links ]
Received on: November 22, 2007
Accepted on: August 25, 2008
Address reprint requests to: BSC Gabriela Salmón Mulanovich. Centro Médico Naval-NMRCD. Av. Venezuela cdra. 36 s/n, Bellavista, Callao 2. Perú.
E-mail: gabriela.salmon@med.navy.mil














