Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Salud Pública de México
versión impresa ISSN 0036-3634
Salud pública Méx vol.46 no.3 Cuernavaca may./jun. 2004
ARTÍCULO ORIGINAL
The logistic model for predicting the non-gonoactive Aedes aegypti females
El modelo logístico para predecir la frecuencia de hembras no gonoactivas en función del tamaño corporal y tipo de colecta, en poblaciones de Aedes aegypti, en Monterrey, México
Filiberto Reyes-Villanueva, PhDI; Mario A Rodríguez-Pérez, PhDII
IFacultad de Ciencias Biológicas, Laboratorio de Entomología Médica, Universidad Autónoma de Nuevo León. San Nicolás de los Garza, Nuevo León, México
IICentro de Biotecnología Genómica, Instituto Politécnico Nacional. Reynosa, Tamaulipas, México
ABSTRACT
OBJECTIVE: To estimate, using logistic regression, the likelihood of occurrence of a non-gonoactive Aedes aegypti female, previously fed human blood, with relation to body size and collection method.
MATERIAL AND METHODS: This study was conducted in Monterrey, Mexico, between 1994 and 1996. Ten samplings of 60 mosquitoes of Ae aegypti females were carried out in three dengue endemic areas: six of biting females, two of emerging mosquitoes, and two of indoor resting females. Gravid females, as well as those with blood in the gut were removed. Mosquitoes were taken to the laboratory and engorged on human blood. After 48 hours, ovaries were dissected to register whether they were gonoactive or non-gonoactive. Wing-length in mm was an indicator for body size. The logistic regression model was used to assess the likelihood of non-gonoactivity, as a binary variable, in relation to wing-length and collection method.
RESULTS: Of the 600 females, 164 (27%) remained non-gonoactive, with a wing-length range of 1.9-3.2 mm, almost equal to that of all females (1.8-3.3 mm). The logistic regression model showed a significant likelihood of a female remaining non-gonoactive (Y=1). The collection method did not influence the binary response, but there was an inverse relationship between non-gonoactivity and wing-length.
CONCLUSIONS: Dengue vector populations from Monterrey, Mexico display a wide-range body size. Logistic regression was a useful tool to estimate the likelihood for an engorged female to remain non-gonoactive. The necessity for a second blood meal is present in any female, but small mosquitoes are more likely to bite again within a 2-day interval, in order to attain egg maturation. The English version of this paper is available too at: http://www.insp.mx/salud/index.html
Key words: Aedes aegypti; dengue; wing-length; non-gonoactive; Mexico
RESUMEN
OBJETIVO: Estimar la probabilidad para que una hembra de Aedes aegypti, previamente alimentada con sangre humana, permanezca no gonoactiva, sin madurar huevos, dependiendo del tamaño corporal y tipo de colecta.
MATERIAL Y MÉTODOS: Se hicieron 10 muestreos de Ae aegypti.: seis de hembras capturadas en cebo humano, dos de nulíparas y dos colectadas en reposo intradomiciliar. Cada muestreo incluyó 60 hembras, en tres colonias endémicas para dengue, en Monterrey, Nuevo León, México, entre 1994 y 1996. Las hembras grávidas o con sangre en estómago fueron excluidas. Cada mosquito fue llevado al laboratorio y alimentado a repleción con sangre humana, y a las 48 horas los ovarios fueron disecados para registrar si estaban en fase gonoactiva o no. El tamaño corporal fue registrado por la longitud en mm del ala izquierda de cada mosquito. Se usó regresión logística para estimar la probabilidad de que una hembra fuera no gonoactiva, como una variable binaria, en función de la longitud alar, y del tipo de colecta.
RESULTADOS: De las 600 hembras procesadas, 164 (27%) permanecieron no gonoactivas, y su tamaño corporal mostró un intervalo (1.9-3.2 mm) casi igual al total de las hembras (1.8-3.3 mm). El modelo de regresión logística fue significativo para estimar la probabilidad de que una hembra permanezca no gonoactiva (Y=1). El tipo de colecta no tuvo influencia significativa en la variable binaria, pero la probabilidad de no gonoactividad mostró una relación inversa con el tamaño corporal.
CONCLUSIONES: Las poblaciones del vector del dengue en Monterrey, México, están integradas por hembras de tamaño muy variable. La regresión logística resultó útil para evaluar la probabilidad que tiene una hembra de quedar no gonoactiva después de una alimentación sanguínea. La necesidad de una segunda comida sanguínea está presente en cualquier mosquito, pero los pequeños tienen una mayor probabilidad de picar por segunda ocasión a una persona en un periodo de dos días, para poder madurar huevos. El texto completo en inglés de este artículo también está disponible en: http://www.insp.mx/salud/index.html
Palabras clave: Aedes aegypti; dengue; longitud alar; no gonoactiva; México
The body size of Aedes aegypti has been associated with its vectorial competence for some viral strains of dengue (DEN) virus.1 It has been reported that although Ae aegypti sometimes ingests plant carbohydrates,2 it prefers to feed only on human blood.3 This inclination in wild mosquitoes makes the females more prolific and long-lived than those reared on blood plus sugar.4 The water-holding containers found in urban habitats that serve as breeding sites for a varying number of mosquitoes typically undergo changes of water volume, consequently, the amount of food can also vary causing an effect on the nutritional larval stage and the body size of the adult mosquito. The newly emerged females can be divided into Christophers stages,5,6 in an ordinal scale to measure the ovarian development extent. Christophers stages I, IIb, and III-V correspond to three oogenic phases: the previtellogenic, resting (quiescent), and vitellogenic phase.7 Most newly emerged Ae aegypti females have their ovaries in stage IIb; however, malnourished mosquitoes with their ovaries in stage I can also be found.8,9 There have been a few through studies that have examined the association between the body size of domestic mosquitoes and those that remain non-gonoactive, i.e., with neither egg maturation nor oviposition, after ingesting a human blood meal to repletion. In an important work,8 colony-reared Ae aegypti females in stage I needed two successive blood meals to accomplish ovarian maturation. A first blood meal in mosquitoes allowed the development of the ovaries from stage I to II, and the full egg development occurred after a consecutive second blood meal. Nutritionally weak Anopheline mosquitoes requiring two complete blood meals for full egg development were defined as "pregravid females".10,11 Although a "pregravid phase" is a common event in the oogenesis of Culicidae mosquitoes, the term has not been widely accepted by mosquito biologists.12 In this paper, a "non-gonoactive female" will be more correctly referred to as a mosquito in Sella stage I, i.e., with its blood meal entirely digested,6 and with its ovarian development, after engorgement, not beyond Christophers stage IIa.7 Thus, we assumed that a non-gonoactive female used up all nutrients of the first blood meal to increase its caloric reserve level and the oogenesis reached only Christophers stage IIa. It has been reported that mosquitoes with ovarian development below stage IIa cannot develop eggs.7
Ae aegypti laboratory females with a wing length shorter than 2.9 mm needed two blood meals to reach the vitellogenic phase.9 It has also been reported that Anopheles albimanus and An gambiae s.l. with a wing length <2.9 mm did not develop eggs after ingesting their first blood meal.13 However, small females could become gonoactive and large ones non-gonoactive. The purpose of this study was to evaluate the logistic model for predicting the frequency of non-gonoactive females, as a dependent variable of body size and collection type in wild Ae aegypti populations. Different types of collections of biting, resting, and container-emerging mosquitoes were performed to ensure the capture of the widest female body size range naturally occurring. The wing length and Christophers stages of wild Ae aegypti mosquitoes after taking a full human blood meal were determined. These data were used to develop a binary logistic regression model to estimate the likelihood of a female mosquito being non-gonoactive P(GI=1) or not (GI=0) considering the wing length X1 and the collection category X2 as the explanatory covariates in the global model.
Material and Methods
Aedes aegypti females were collected in three dengue endemic neighborhoods in Monterrey: Francisco I. Madero, Lázaro Cárdenas, and El Mirador. Dwellings were clustered and built with cement and metallic or cement roofs. In the first neighborhood, containers including cans, bottles, and discarded tires were abundant in back yards, while in the other two, 200-liter drums were commonly used by homeowners as water-storage containers due to the lack of piped water. The climate in Monterrey is arid with a mean annual rainfall in the area of 450 mm (range=270 mm-620 mm), average temperature of 23 ºC (range=-2 ºC -44 ºC) and relative humidity of 60% (range=32-90%). The two rainy months are May and October,14 and the highest population densities of Ae aegypti occur in these months.15
Ten collection sets of mosquitoes were performed from 1994 through 1996. Six human-biting collections (one in October 1994, May 1995, October 1995, and three in October 1996) were conducted in five houses at Francisco I. Madero. Two collections of container-emerged mosquitoes were done in October 1994 from five drums at El Mirador, and from five tires at Lázaro Cárdenas. Finally, two indoor-resting collections were conducted in five houses at El Mirador in May 1995, and October 1995. Each collection set consisted of 60 females. Each human-biting collection of 60 mosquito females was carried out in a 5-day capture interval by a two-person team (the attractant and collector catching mosquitoes on one person). Mosquitoes were captured in backyards from 17:00 to 20:00 h with a mouth aspirator immediately after they posed on the legs and arms of one of the authors posing as the volunteer human bite. The mosquitoes were held in a cardboard cage and transported to the laboratory the same day of capture for an examination of their abdomen, after being anesthetized by a 10-minute exposure inside a freezer (~ -2 ºC). Females with the abdomen completely empty were separated for blood feeding, whereas those with blood vestiges in stomach and/or gravids were removed. Twenty-four hours after their capture and kept only with access to water, empty females were fed to repletion on the hand of one of the authors who volunteered to do so. Mosquitoes were not interrupted during blood feeding, which lasted until they withdrew their mouthparts freely from the volunteer's hand. Forty-eight h after blood feeding, mosquitoes were immobilized by freezing and the ovaries of the females that had fed to repletion were dissected in 0.5% saline solution and observed through a stereomicroscope to determine the Christophers stage of the oocyte in development.5,6 The wing of each mosquito was excised and the wing length measured from the axillary incision to the apical margin, excluding the fringe scale.16 As we mentioned before, a non-gonoactive female was one that fed to repletion 48 h prior to examination with blood completely digested, and ovaries not beyond Christophers stage IIa.7 On the contrary, a female previously fed to repletion and with ovaries in any stage beyond IIIa, 48 h after blood feeding and blood completely digested was considered to be a gonoactive female, i.e. with eggs in maturation.
Each collection of 60 resting mosquitoes was conducted inside the houses in a 5-day interval. This sample size was chosen considering that 30 is the limit between small and large samples.17 The same five houses were sampled for each collection. For emerging female collections, pupae were collected from five 200-liter drums and five discarded tires to obtain teneral females. A total of 150 pupae from drums, and 150 pupae from tires were collected and transported to the laboratory where they were placed into screened emergence cages (30 x 30 x 30 cm). Here, mosquitoes had access to a 10% sucrose solution provided in cotton pads, and newly emerged females and males were allowed to mate. After 72 hours of emergence the volunteer author introduced a hand into each cage to feed the mosquitoes to repletion. Time feeding varied among individuals because each female was allowed to feed until she withdrew freely her proboscis from the skin. Twenty-four hours post-blood feeding, 60 engorged females were placed into a new cage, and 48 h after her blood meal, the ovaries were dissected and the wing length of each female was measured as above.
Statistical analysis
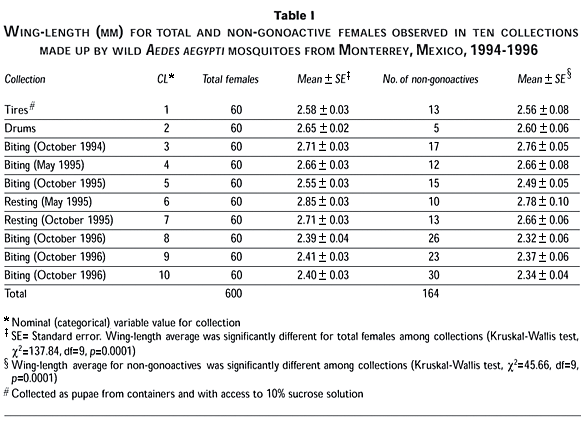
The 600 wing length data were sorted in ascending order, and a histogram was built. Lowest and highest limits of the entire wing length range were 1.8 and 3.3 mm, comprising 16 classes of 0.1 mm width each, where the 2.5 mm class was identified as the median. Total and non-gonoactive female frequencies were represented in this histogram.
The arithmetic mean and standard error of the wing length for each collection were calculated. Variation in wing length average for the total number of mosquitoes and non-gonoactive mosquitoes among collections was compared by a Kruskal-Wallis test using procedures Proc means and Proc npar1way in SAS.18 A dataset of 600 values made up of three variables: non-gonoactive (GI=the response variable; a binary-discrete variable), wing length (WL; a continuous variable), and collection category (a nominal variable) was constructed. A logistic regression model was applied using procedure Proc logistic in SAS19 to these data, using the binary variable GI as the dependent variable (Y) and the other two as the covariates (X). The nominal variable or "collection category" (CL) was arranged in values from 1 to 10 according to the collection number (Table I). A linear logistic regression model was transformed to the exponential form by calculating the antilog (ex) of each linear parameter, and the likelihood of being non-gonoactive P(GI=1) was estimated according to WL and CL values for each female mosquito. The probability values were plotted on the Y-axis against the total wing length range in the X-axis. Finally, with the options: descending and lackfit in the proc logistic procedure in SAS, the number of observed and expected non-gonoactive females per wing length decile (Q10 in the entire probability scale) was calculated and compared using the c2 statistic of the Hosmer-Lemeshow test.20
Results
One hundred and sixty four out of 600 Ae aegypti mosquitoes (27%) that were fully fed and dissected from ten collections carried out during 1994-1996 remained non-gonoactive, because they showed their ovaries in Christophers stage I, II or IIa. Most of the (108/164=66%) non-gonoactive females presented Christophers stage II; 53/164=32% exhibited the previtellogenic stage I, and only 3/164=2% had ovaries in stage IIa.
Non-gonoactive females wing length ranged from 1.9 to 3.2 mm within the wing length entire range of 1.8-3.3 mm observed for all mosquitoes collected in this study (Figure 1). A total of 74% of the non-gonoactive mosquitoes were concentrated in three wing length classes on each side of the median=2.5 mm. Thus, there were 122 non-gonoactive females in the 2.2-2.8 mm range. In addition, 23 (14%) non-gonoactive mosquitoes had the smallest wing length (range=1.9-2.1 mm), and 19 (12%) had the largest one=2.9-3.2 mm, although there was none in the 3.1 mm class. Among the largest non-gonoactives, 17 (10% out of 164) had a wing length longer than 2.9 mm (Figure 1). These largest non-gonoactive mosquitoes were obtained from four human-biting collections and from resting captures.
In relation to collection date, the biting capture of 1996 resulted in the highest number of non-gonoactive females (16%, 14%, and 18%) in comparison with 3% of emerging females from water drums, 8% from tires, and 6% and 8% from resting captures. The Kruskal-Wallis test showed that the wing length average of the non-gonoactive mosquitoes varied among collections (c2=45.66, df=9, p=0.0001); a similar finding was observed for the wing length average of total females among collections (c2=137.84, df=9, p=0.0001) (Table I). Moreover, the smallest non-gonoactive females (2.32±0.06 mm) corresponded to the biting collections of 1996. The non-gonoactive females collected as pupae from tires were, in general, at the middle of the size range of 2.56±0.08 mm, whereas the largest non-gonoactive mosquitoes of 2.78±0.10 mm were found in resting collections (Table I).
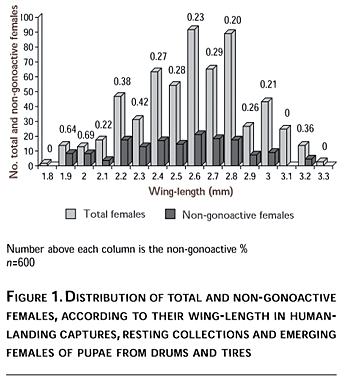
The overall logistic model to calculate the probability of occurrence of a non-gonoactive female P(GI=1; as a binary variable) was highly significant (p=0.0001) according to a likelihood-ratio c2 test (-2 LOG L) with 1 df. The model accurately predicted 65% of the responses, and the Nagelkerke's R2 (max-rescaled R2) was 0.09. The Wald c2 statistic showed significance for the slope of both covariates, indicating that the WL as well as the CL had a significant influence on the probability of incidence of a non-gonoactive female in a sampling scheme (Table II, Figure 2). A negative slope (-1.02) for WL means that P(GI=1) decreases when the WL increases. According to the slope values for both covariates, the WL had a significant effect [(1/exp(b)=2.77] shown by the odds ratio, and two-fold higher in comparison to the CL variable [(1/exp(b)=1.15].

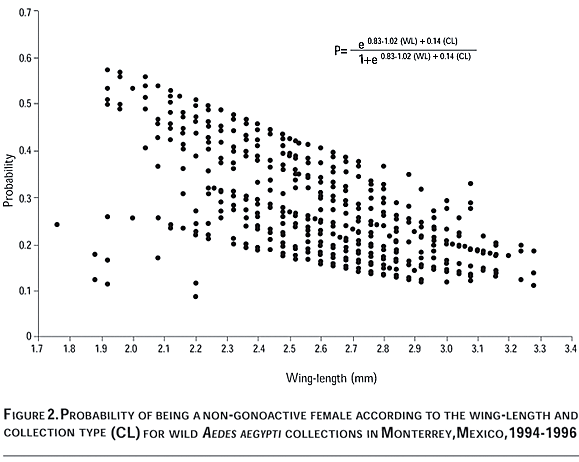
Finally, the observed and expected number of non-gonoactive females per decile (Q10) along the whole sorted probability scale were similar according to the Hosmer-Lemershow goodness of fit test (c2=13.22, 8 df, p=0.10), and these values were also similar to the true number of non-gonoactive mosquitoes collected in this study (Figure 3).
Discussion
A body-size threshold of 2.9 mm, below which Ae aegypti laboratory-raised females remain non-gonoactive after engorging on human blood,9 does not coincide with the wing length range found in wild non-gonoactive populations of the dengue vector in Monterrey, Mexico. In our results, 10% of the non-gonoactive females, which was approximately 3% of the total number of collected mosquitoes of 600, had a large body size > 2.9 mm. Further examination of the Feinsod and Spielman9 data revealed a similar result because they also observed large mosquitoes that failed to mature eggs after their first human blood-engorgement. In one experiment, they engorged 65 Ae aegypti females, out of which 11 (17%) remained non-gonoactive, yet had a wing length of 3.0 mm. In Anopheles, the scenario does not seem different because An arabiensis females that stayed non-gonoactive had a mean wing length smaller than 2.97 ± 0.014 mm.21
A large female may not necessarily be a well-fed mosquito, and she will not always become gonoactive after her first blood meal. The amount of lipids deposited in Ae aegypti ovaries from blood meals was similar in either small or large females, but glycogen levels were higher in small mosquitoes.22 This strategy has evolved to improve survival, but compels small females to take a second blood meal before egg-laying.
This could explain our observations: overall, the higher the mean wing length in our mosquito collection, the lower the non-gonoactive female number in that collection (Table I).
Our results suggest that the presence of large Ae aegypti non-gonoactive females is usual in wild Aedes aegypti populations. Regardless of the terms, there is a common trait between the "pregravid females" of Gillies11 and the non-gonoactive females found in this study: both require at least a second blood meal to mature eggs. In addition, it has been pointed out that in this species, there is always a proportion of "pregravid" females varying from 5% to 10%, regardless of both larval density and food amount during larval breeding.8 Indeed, MacDonald8 also reported that in a small indoor-resting collection of 33 Ae aegypti mosquitoes in Malaya, eight (24 %) did not develop eggs after ingesting a replete human blood meal.8 A similar proportion has been reported in Ae albopictus field populations, in which 20% was the highest incidence of non-gonoactive mosquitoes estimated from multiple blood meals per gonotrophic cycle.23 Our results revealed that the percentage of non-gonoactive females that could be involved in three blood meals to repletion, including the last one required to initiate the next gonotrophic cycle, was approximately 32% (those in stage I) of the total number of non-gonoactive females, and around 4% of the whole capture. Similarly, non-gonoactive females implicated in two blood meals comprised around 68% (those in stage II and IIa) of the total number of non-gonoactive females. Therefore, non-gonoactive mosquitoes in stage I from Monterrey comprised a population that must ingest three blood meals in the same gonotrophic cycle. Highly competent mosquitoes with multiple feedings increase the human-vector contact rate, thus they may be associated with endemic areas where the four serotypes of the DEN virus may be in circulation. It seems that a few non-gonoactive females feeding indoors would be enough to produce a dengue outbreak.
In conclusion, the prediction power of the logistic regression to estimate the probability P(GI=1) of being a non-gonoactive wild Ae aegypti mosquito, demonstrated to be an acceptable tool for field surveys. These surveys could include biting, emerging, and resting mosquitoes, and the logistic model will predict the frequency of non-gonoactive females in the collections, with a known probability level, and in function of the wing length and collection category as explanatory variables.
Acknowledgments
We express our gratefulness to Carolina Briceño Dávila who was the responsible for dissection of most mosquitoes processed in this study. We also thank to Dra. Olga Najarro from Universidad Valle de Bravo, Tamaulipas, México, for an early review of the draft, and to the anonymous reviewer of Salud Pública de México by relevant corrections done in the whole manuscript.
References
1. Sumanochitrapon W, Strickman D, Sithiprasasna R, Kittayapong P, Innis BL. Effect of size and geographic origin of Aedes aegypti on oral infection with dengue-2 virus. Am J Trop Med Hyg 1998;58:283-286. [ Links ]
2. Martínez-Ibarra JA, Rodríguez MH, Arredondo-Jiménez JI, Yuval B. Influence of plant abundance on nectar feeding by Aedes aegypti (Diptera: Culicidae) in southern México. J Med Entomol 1997;34: 589-593. [ Links ]
3. Scott TW, Naksathit A, Day JF, Kittayapong P, Edman JD. A fitness advantage for Aedes aegypti and the viruses it transmits when females fed only on human blood. Am J Trop Med Hyg 1997;57:235-239. [ Links ]
4. Costero A, Edman JD, Clark GG, Scott TW. A life table study of Aedes aegypti (Diptera: Culicidae) in Puerto Rico fed only human blood versus blood plus sugar. J Med Entomol 1998;35:809-813. [ Links ]
5. Christophers SR. The development of the egg follicle in anophelines. Paludism 1911;2:73-88. [ Links ]
6. World Health Organization. Manual on practical entomology in malaria. Part II. Methods and techniques. Ginebra WHO; 1975. (WHO Offset Publication 13). [ Links ]
7. Clements AN. The biology of mosquitoes. Vol. 1. Development, nutrition and reproduction. London: Chapman & Hall; 1992. [ Links ]
8. MacDonald WW. Aedes aegypti in Malaya. II. Larval and adult biology. Ann Trop Med Parasitol 1956;50:399-414. [ Links ]
9. Feinsod FM, Spielman A. Nutrient-mediated juvenile hormone secretion in mosquitoes. J Insect Physiol 1980;26:113-117. [ Links ]
10. Gillies MT. The recognition of age-groups within populations of Anopheles gambiae by the pre-gravid rate and sporozoite rate. Ann Trop Med Parasitol 1954;49:320-325. [ Links ]
11. Gillies MT. The pre-gravid phase of ovarian development in Anopheles funestus. Ann Trop Med Parasitol 1955;49:320-325. [ Links ]
12. Washino RK. The physiological ecology of gonotrophic dissociation and related phenomena in mosquitoes. J Med Entomol 1977;13:381-388. [ Links ]
13. Lyimo EO, Takken W. Effects of adult body size on fecundity and the pre-gravid rate of Anopheles gambiae s.l. females in Tanzania. Med Vet Entomol 1993;7:328-332. [ Links ]
14. Instituto Nacional de Estadística, Geografía e Informática. Carta Geográfica del Estado de Nuevo León. México DF: Gobierno Federal, Secretaría de Programación y Presupuesto; 1986. [ Links ]
15. Salas-Luévano M, Reyes-Villanueva F. Variación estacional de las poblaciones de Aedes aegypti en Monterrey, México. Salud Publica Mex 1994;36:385-392. [ Links ]
16. Xue RD, Edman JD, Scott TW. Age and structure effects on blood meal size and multiple blood feeding by Aedes aegypti (Diptera: Culicidae). J Med Entomol 1995;32:471-474. [ Links ]
17. Zar JH. Biostatistical analysis. Prentice-Hall; 1974. [ Links ]
18. Schlotzauer SD, Littel RC. SAS system for elementary analysis. Cary (NC); SAS Institute; 1987. [ Links ]
19. SAS. Logistic regression examples using the SAS system, version 6. Cary (NC): SAS Institute; 1995. [ Links ]
20. Hosmer DW, Lemeshow S. Applied logistic regression. John Wiley & Sons; 1989. [ Links ]
21. Hogg JC, Thompson MC, Hurd H. Comparative fecundity and associated factors for two sibling species of the Anopheles gambiae complex occurring sympatrically in The Gambia. Med Vet Entomol 1996;10:385-391 [ Links ]
22. Naksathit AT, Edman JD, Scott TW. Amounts of glycogen, lipid, and sugar in adult female Aedes aegypti (Diptera: Culicidae) fed sucrose. J Med Entomol 1999,36:8-12. [ Links ]
23. Hawley WA. The biology of Aedes albopictus. J Am Mosq Control Assoc 1988;(Suppl 1):40. [ Links ]
Adress reprint requests to
Dr. Filiberto Reyes Villanueva
Universidad Autónoma de Nuevo León
Facultad de Ciencias Biológicas
Laboratorio de Entomología Médica
P.O. Box 109-F, 66450 San Nicolás de los Garza, Nuevo León, México
E-mail: frvi@prodigy.net.mx
Received on: july 24, 2003
Accepted on: march 5, 2004














