Services on Demand
Journal
Article
Indicators
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Salud Pública de México
Print version ISSN 0036-3634
Salud pública Méx vol.45 suppl.3 Cuernavaca Jan. 2003
ARTICLE ARTÍCULOS
Prospects for primary prevention of cervical cancer in developing countries
Perspectivas de prevención primaria de cáncer cervical en países en desarrollo
Silvia Franceschi, MD; Gary Clifford, Ph D; Martyn Plummer, MA
International Agency for Research on Cancer. Lyon, France
ABSTRACT
The HPV types that cause cervical cancer are sexually transmitted, but there is little evidence that infection can be avoided by behavioural changes, such as condom use. In contrast, prophylactic vaccines against HPV infection are likely to have high efficacy. In principle, the effectiveness of HPV vaccination as a strategy for cervical cancer control can be measured either by monitoring secular trends in cervical cancer incidence or by conducting randomized trials. The former approach is unlikely to provide convincing evidence of effectiveness, since cervical cancer rates are subject to strong secular trends that are independent of intervention measures. A few phase III trials of HPV prophylactic vaccines are now being started. Such trials are very expensive studies involving frequent and complicated investigations. It is important, however, to start as soon as possible simpler trials designed to demonstrate the effectiveness of HPV vaccine in field conditions, i.e. in developing or intermediate countries which suffer the major burden of mortality from cervical cancer. Such trials may capture a difference in the most severe, and rarest, preinvasive cervical lesions (i.e., the real target of any HPV vaccine) over a prolonged follow-up (20 years at least). The design of such studies is briefly considered for two areas: Southern India and South Korea. This paper is available at: http://www.insp.mx/salud/index.html
Key words: cervix neoplasms; vaccination; randomized controlled trials; projection
RESUMEN
Los tipos de virus de papiloma humano (VPH) que causan cáncer cervical son sexualmente transmisibles, pero existe muy poca evidencia sobre que la infección pueda ser evitada por cambios en las conductas sexuales de alto riesgo, tales como el uso del condón. En contraste, vacunas profilácticas en contra del VPH pueden llegar a tener una muy elevada eficacia en la prevención de cáncer cervical. En principio, la efectividad de la vacunación contra el VPH, como estrategia para el control de cáncer cervical, puede ser evaluada por monitoreo secular en las tendencias de incidencia de cáncer cervical o mediante la conducción de ensayos clínicos aleatorizados. El primer tipo de estudios no puede demostrar en forma convincente su efectividad, porque las tasas de incidencia y mortalidad por cáncer cervical son influenciadas fuertemente por tendencias seculares que son independientes de las medidas de intervención. Estudios de fase 3 de vacunas profilácticas contra el VPH están siendo desarrollados actualmente. Cada uno de los ensayos es muy costoso e involucran complejos tipos de diseño. Esto es importante, sin embargo, deben iniciarse, tan pronto como sea posible, ensayos más simples diseñados para demostrar la efectividad de una vacuna contra el VPH en condiciones de campo, como las que pueden existir en países en desarrollo, donde el peso de la mortalidad por cáncer cervical es muy alto. Cada ensayo clínico puede cuantificar una diferencia en la presentación de lesiones precursoras de cáncer (el real blanco de una vacuna contra el VPH) en un periodo de seguimiento prolongado (20 años, al menos). En este artículo se presenta brevemente el posible diseño de este tipo de estudios, considerando dos áreas: India meridional y Corea del Sur. Este artículo también está disponible en: http://www.insp.mx/salud/index.html
Palabras clave: vacuna; ensayos clínicos; cáncer cervical; virus de papiloma humano; proyecciones
Developing countries have both higher rates of invasive cervical cancer (ICC) and poorer survival from ICC than developed countries. More than 80% of deaths from ICC occur in developing countries, most notably in Latin America, India, and Sub-Saharan Africa.1 Women in developing countries do not have access to screening programmes, which have successfully reduced the incidence of cervical cancer in many developed countries. There are considerable obstacles to setting up effective screening programmes in low resource settings.2,3 Alternatives to cytology-based screening that are more appropriate for low resource settings are currently being explored,4,5 but the most promising means of reducing the global burden of cervical cancer is the control of HPV infection, which is the central cause of cervical cancer.6,7 Control of HPV may be attempted through both non-specific interventions, such as behavioural modification, and specific interventions such as immunization.
Primary prevention of HPV
The demonstration that ICC is caused by a sexually transmitted virus (i.e., human papillomavirus)6,7 adds ICC to the list of severe diseases (AIDS tops the list) where "safe sex" educational campaigns should have a role to play. Some specific characteristics of HPV infections make it, however, an especially difficult target for this form of intervention.
Firstly, HPV infection is very common: at any moment in time between 5% and 40% of adult women and men are HPV carriers.8-10 Except for genital warts (caused chiefly by low-risk types 6 and 116), the infection is asymptomatic. Indeed, an association between HPV infection and the number of sexual partners has been found consistently, but it is weak, on account of the high probability of exposure with any given partner.9,11 There is no clear evidence as yet that barrier methods of contraception, most notably condoms, confer a protection against HPV infection.10,11 The apparent failure of condom use to prevent HPV infection may be attributable to anatomic reasons (i.e., extension of HPV infection to genital areas not protected by the condom) and behavioural reasons (i.e., difficulty of using condoms consistently over long periods, especially in stable couples). Only circumcision was found associated with a decreased risk of HPV penile infection among men and cervical cancer among their wives in a large multi-centric study conducted by the IARC in five countries.12
An alternative strategy to prevent ICC may be trying to intervene on those factors which are known to facilitate the persistence of HPV infection or the progression into neoplastic cervical lesions. Such factors include immune suppression,6 multi-parity,13 long-term use of oral contraceptives,14 cigarette smoking15 and some sexually transmitted diseases other than HPV (i.e., HSV-2,16 and Chlamydia trachomatis17). In contrast to HPV, however, these risk factors are relatively weak (relative risks of 2 to 3) and are difficult to eliminate for the purpose of ICC prevention.
Vaccines against HPV offer by far the best hope of controlling HPV infection. A vaccine that is safe, effective and cheap would provide an opportunity to diminish the health inequality that currently exists between developed and developing countries with respect to ICC.
Vaccines against HPV
HPV vaccines currently under development are part of a new generation of vaccines that employ genetic engineering.18 This allows the production of sub-unit vaccines that include only a portion of a disease-causing organisms; since they do not contain cancer-inducing viral genes, these may be safer than vaccines based upon whole organisms.
Several therapeutic vaccines against HPV are under investigation.18 They target, in general, transforming viral proteins such as E6 and E7, and are being tested in early phase II trials against advanced ICC, as a complement to conventional treatment.
Prophylactic vaccines against HPV infection seem, at the moment, more promising than therapeutic ones, and several different approaches are being investigated (e.g., recombinant live vector vaccines, protein and peptide vaccines, naked DNA vaccines and edible vaccines). The most advanced vaccines against HPV are virus-like particles (VLPs). VLPs result from the ability of the viral capsid proteins L1 and L2 (or L1 alone) to self-assemble into particles which are empty, but closely ressemble the entire virus and include conformational epitopes that induce virus-neutralizing antibodies.19 VLPs have been produced for at least ten HPV types (6, 11, 16, 18, 31, 33, 35, 39, 45, and 58), suggesting that this approach can be applied for a multivalent vaccine.18
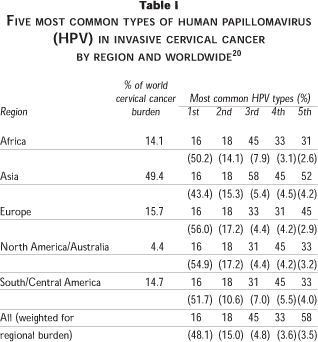
The protection conferred by VLP vaccines is type-specific. Over 90 types that infect the genital tract have been identified, of which approximately 20 are currently considered "high-risk" types for cervical cancer. Of these "high-risk" types, a meta-analysis of 10 058 ICC cases from 50 different countries has shown that HPV types 16 and 18 alone contribute to at least 65% of cervical cancers worldwide, and that HPV types 45, 31 and 33 contribute to another 10-15%.20 Table I shows the five most common HPV types in different regions as derived from this meta-analysis. Fortunately, for most regions of the world sampled, it appears that the same five "high-risk" types predominate in cervical cancer, although there is little information on types other than 16 and 18 for many regions (Africa, India, Middle East), and HPV 52 and 58 appear to contribute a larger proportion of cases from areas in Asia. Thus, a vaccine that protects against relatively few HPV types has the potential to prevent a large proportion of cervical cancers worldwide.
Independent phase III randomized clinical trials of L1 VLP vaccines are currently being started by the National Cancer Institute (NCI) of the United States, Merck and GlaxoSmithKline. In each trial, the vaccine is administered as a series of three injections (generally at month 0, 1 and 4).18 The majority of the populations involved in these trials are from the United States and Latin America. All current trials use vaccines that include VLPs of both HPV 16 and 18.
An average of 10 000-15 000 young women (age range: 18-22) will be recruited in each such trial, the aim being to vaccinate as many women not yet exposed to genital HPV as possible. In fact, a prophylactic vaccine needs to be administered before a woman has been infected. Ideally, the vaccine should be given to children where immunization is logistically easier. The present trials, however, are targeting young women in order to be able to monitor the efficacy of an HPV vaccine in a reasonable time framework (approximately five years). Adequate study endpoints for such trials are still being discussed, but they will include, in the order, the prevention of HPV infection, persistent infection (i.e., repeated HPV-positive smears at a 6-12 month interval), and cervical lesions (i.e., low- and high-grade squamous intraepithelial lesions, LSIL and HSIL).
For the moment, the findings on the efficacy of VLP vaccines in animals and humans have been very encouraging. Three injections of L1 VLPs protected rabbits, dogs and cattle against persistent infection and carcinomas caused by species-specific papillomavirus.18
In humans, a double-blind, placebo-controlled trial of 72 volunteers (58 females and 14 males) showed that the HPV 16 VLP vaccine developed at the NCI is well tolerated and highly immunogenic, even without adjuvants.21 In the majority of recipients serum antibody titers that were approximately 40-fold higher than those observed in the natural infection were achieved.
In a double blind study of 1533 HPV 16-negative young women, who were followed for 17.4 months on average, the incidence of persistent HPV 16 infection was 3.8 per 100 women-years in the placebo group and 0 per 100 women-years in the vaccine group (100 percent efficacy).22
The need for effectiveness trials
Immediate uptake of any HPV vaccination worldwide following licensure of a vaccine is unlikely. Furthermore, it will take a long time for the impact of HPV vaccination to become visible in vaccinated populations. Even then, the impact of vaccination may be confounded by changes in the rate of cervical cancer so that secular trends in cervical cancer rates may not be easily interpretable as evidence for the effectiveness of vaccination. There is therefore scope for planning long-term randomized trials of HPV vaccines. These trials will differ from the phase III efficacy trials that are currently taking place in several respects. In particular, they may take place under field conditions likely to prevail when a real vaccine programme is introduced, and will have to include a longer follow up period (typically more than 10 years) in order to show the efficacy of the vaccine against truly precancerous lesions such as cervical intra-epithelial neoplasia (CIN) III.
When considering the impact of a vaccine on cancer incidence, it is useful to consider past experience with hepatitis B virus (HBV).23 Like HPV, HBV is a cause of cancer (hepatocellular carcinoma) in chronically infected individuals. Unlike HPV, however, HBV is also associated with acute disease at the time of infection and substantial morbidity and mortality from causes other than cancer.24 These other factors were sufficient to motivate the development and spread of vaccines against HBV, which proved effective in preventing acute hepatitis and the chronic carrier state. Questions remained over the duration of protection and, in particular, the extent to which vaccination would prevent hepatocellular carcinoma.
Two different approaches have been used to answer this question: conducting randomized trials and following secular trends. The Gambia Hepatitis Intervention Study25 is a good example of a large randomized trial of HBV vaccine. This is a cluster-randomized trial of HBV vaccination and liver cancer based on the phased introduction of HBV vaccination into the Extended Programme of Immunization (EPI) in The Gambia between 1986 and 1990. Early results from the study showed that vaccination has an efficacy of 83% against infection and 95% against chronic carriage of HBV.26 The results for liver cancer are not yet available since the subjects have not reached the high-risk age range: the study was originally planned to last 30 to 40 years. The second approach of following secular trends is illustrated by Taiwan, where a national vaccination programme against HBV was introduced in 1986. A substantial reduction in childhood liver cancer incidence is already visible. The incidence rate in children aged 6-14 was 0.70 per 100 000 in 1981-1986 and 0.36 per 100 000 in 1990-1994 (p<0.01).27
In the case of HPV vaccination, it is unlikely that secular trends in cervical cancer incidence or mortality will provide convincing evidence of effectiveness since cervical cancer incidence shows considerable temporal variation. A decline in incidence and mortality from cervical cancer has been observed in many populations before the introduction of screening programmes.28 The decline may be attributable to a decrease in early and frequent child bearing,13 to improved nutrition or genital hygiene, or to other factors related to sexual behaviour. Conversely, in some developed countries, most notably in the United Kingdom, the "sexual revolution" of the 1960's has led to an epidemic of HPV infection and subsequent increase in ICC incidence and mortality in young women.28
Design considerations for effectiveness trials
In order to provide direct evidence of the effectiveness of HPV vaccination in preventing cancer, simple randomized trials should be undertaken. Such trials could be conducted in any country, but in order to accelerate adoption of HPV vaccination in the populations that most need it, priority should be given to developing countries, most notably Asia where 50% of ICC worldwide cases occur. These trials should be large and of long duration (20 years at least) in order to capture a difference in the most severe preinvasive cervical lesions, which take many years to develop. Consequently, the design must be simple and cost-effective. The trial design may be summarized as follows:
- Vaccination of young women before they become exposed to HPV (i.e., in conservative societies, before marriage).
- Long-term "opportunistic" monitoring of side effects (e.g., through monitoring of hospital admissions).
- No measurement of cervical outcomes until the subjects are of sufficient age to benefit from screening.
A fundamental difference between this trial and the phase III trials currently being conducted is that there are no plans for early gynaecological examination for the purposes of the trial only. The lack of early examination is beneficial to the participants since women under the age of 30 have a very low risk of cervical cancer and CIN III, but may undergo over-treatment of transient HPV infections which may manifest themselves clinically as CIN I. A corollary of the lack of early gynaecological examination is that a population-based screening programme must be in place for the study participants in due time (e.g., when they reach the age of 30-35). This will ensure two things: Firstly that the control group receives an adequate standard of care, secondly that an outcome measurement, at the age when CIN III peaks, is taken for as many subjects as possible. A second requirement for this study is the ability to follow-up subjects over a long period, and in particular to accurately identify the treatment group decades after randomization.
Possible locations for effectiveness trials
We have considered the possibility of implementing this design in two areas: a rural area in southern India and an urban one in South Korea, which are here presented in respect to their different strengths and weaknesses.
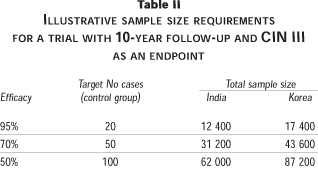
Some areas of Southern India have a very high risk for cervical cancer. The age standardized rate for Chennai (Madras) was 38.9 per 100 000 in the early 1990s.29 This makes it an attractive location for cervical cancer prevention trials. Long-term follow-up of subjects is probably not feasible in an urban setting due to the very marked population movement in developing countries. In a rural setting, the most appropriate study design is a community intervention study with randomization by village. This provides a simple mechanism for identifying the treatment group of a subject many years after randomization, since it suffices to know a subject's place of birth. A cluster randomized trial also presents the only feasible opportunity to randomize males and thus to evaluate the usefulness of vaccinating both sexes. Men very rarely develop severe HPV-related diseases (e.g., cancer of the penis and the anus). They may therefore not respond to individual randomization, but may agree to do so in the context of a community intervention. The efficacy of male vaccination will have to be evaluated in terms of its contribution to the decrease of precancerous lesions in women. To this extent, "discordant" couples (i.e., couples where only the husband or wife is vaccinated) will be most informative. The target population for a trial in Southern India is unmarried women, i.e., women below age 19, as very early marriage is still common in rural areas. Some illustrative sample size calculations are shown in Table II. These are based on an assumption of vaccination at age 15 on a 10-year interval between vaccination and the first screening examination, with CIN III as an endpoint. The incidence rates for CIN are imputed from the incidence rates for invasive cervical cancer by assuming that CIN III occurs five years earlier and at a rate three times higher (hence 2/3 of CIN III will regress without progression to cancer). Loss-to-follow-up has not been factored into these calculations, since a realistic assessment of the rate of loss depends on the specific design of the study. However, in order to take into account loss to follow-up, a possible decline in cervical cancer incidence and the overwhelming difficulty of replicating community-based intervention trials, the target power of the study needs to be very high. These calculations use a target power of 99%, under the assumption that the chance to be able to replicate such huge trials is minimal.
South Korea is no longer considered a developing country but, on account of the recency of the economic and medical development, is still an intermediate-risk country for cervical cancer. The age standardized rate for Busan county, South Korea is 21.8 per 100 000, which is 2-4 fold higher than in most western countries. However, a few characteristics of the population and the health system in South Korea may be greatly beneficial to the implementation of a clinical trial. Age at marriage is substantially older (> 25 years) than in rural India and premarital sexual intercourse is uncommon. In a recent survey coordinated by IARC, all unmarried women below age 20 were negative for anti-HPV antibodies. These characteristics of the population may allow the offering of HPV vaccine to women in the 18-22 year range. A majority of young women in this age range in South Korea attend higher education and may thus be readily contacted, individually randomized, and offered the vaccine in university health facilities. Most importantly, each person in South Korea has a unique national identity number, which will greatly facilitate long-term follow-up. Finally, the South Korean government has a strong commitment to implementing population-based screening programmes in the near future, including cytological screening for the prevention of cervical cancer in women aged 30 or older. Thus, the follow-up process in such a large trial of a prophylactic vaccine against HPV may benefit from the present development of national screening programmes.
Table II also shows illustrative sample size calculations for a trial in Southern Korea. The number of subjects required is 40% larger than the trial in Southern India. The lower incidence rate in South Korea is partially offset by the older age of the study subjects.
Conclusions
Many challenges remain in respect to the efficacy and efficiency of prophylactic vaccines against HPV.
Despite successful results in animal models, it is not clear which elements of the human immune system are important in preventing or resolving HPV infections.30 HPV enters the body through the mucosal membranes and does not spread systemically. Whether the high levels of circulating neutralizing antibodies induced by VLP vaccines translate into an adequate and long-lasting immune response at the mucosal surface is not known. Ways to enhance mucosal immunity and cell-mediated immunity are being evaluated (e.g., intra-nasal or oral immunization).31
Obviously, so-called chimaeric vaccines (i.e., vaccines able to prevent HPV infection and induce clearance of the infection at an early stage) would be a much preferable solution. They would substantially anticipate the benefits of vaccinations that, in the case of prophylactic vaccines, would take three or four decades to become apparent. In fact, therapeutic vaccines may benefit not only sexually inexperienced women who have not yet been infected by HPV, but also older women who may be already harbouring HPV-related cervical lesions.
Furthermore, while safety and efficacy are essential for a vaccine, ways to reduce costs and increase vaccine coverage must also be considered. They will include formulating an oral vaccine, creating a stable vaccine that does not require an expensive cold-chain and/or one that can be produced in developing countries.18 Finally, it is worth bearing in mind that the sexually transmitted nature of HPV infection will probably enter into the public debate, as will the gender issue (i.e., the current restriction of current HPV vaccine to women as a target population). While the efficacy and opportunity of vaccinating boys as well as girls will have to be evaluated, ways to tackle an open discussion on HPV infection will have to be found in developing as well as developed countries.
Notwithstanding the challenges above, HPV vaccine development holds great promises for reducing the mortality and morbidity of cervical neoplasia on the world's women.
References
1. Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 1999;83:18-29. [ Links ]
2. Sherris J, Herdman C. Preventing cervical cancer in low-resource settings. Outlook 2000;18:1-8. [ Links ]
3. Sankaranarayanan R, Budukh AM, Rajkumar R. Effective screening programmes for cervical cancer in low- and middle-income developing countries. Bull World Health Organ 2001;79:954-962. [ Links ]
4. Wesley R, Sankaranarayanan R, Mathew B, Chandralekha B, Aysha Beegum A, Amma NS et al. Evaluation of visual inspection as a screening test for cervical cancer. Br J Cancer 1997;75:436-440. [ Links ]
5. Sankaranarayanan R, Wesley R, Somanathan T, Dhakad N, Shyamalakumary B, Amma NS et al. Visual inspection of the uterine cervix after the application of acetic acid in the detection of cervical carcinoma and its precursors. Cancer 1998;83:2150-2156. [ Links ]
6. International Agency for Research on Cancer. Monographs on the evaluation of carcinogenic risks to humans. Human papillomaviruses. IARC: Lyon, 1995;Vol. 64. [ Links ]
7. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;18:12-19. [ Links ]
8. Herrero R, Hildesheim A, Bratti C, Sherman M, Hutchinson M, Morales J et al. A population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst 2000;92:464-474. [ Links ]
9. Lazcano-Ponce E, Herrero R, Muñoz N, Cruz A, Shah KV, Alonso P et al. Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int J Cancer 2001;91:412-420. [ Links ]
10. Franceschi S, Castellsagué X, Dal Maso L, Smith JS, Plummer M, Ngelangel C et al. Prevalence and determinants of human papillomavirus genital infection in men. Br J Cancer 2002;86:705-711. [ Links ]
11. Molano M, Posso H, Weiderpass E, van den Brule AJC, Ronderos M, Franceschi S et al. Prevalence and determinants of HPV infection among Colombian women with normal cytology. Br J Cancer. 2002;87:324-333. [ Links ]
12. Castellsagué X, Bosch FX, Muñoz N, Meijer C, Shah K, de Sanjosé S, Eluf-Neto J et al. Male circumcision, penile human papillomavirus infection and cervical cancer in female partners. N Engl J Med 2002;346:1105-1112. [ Links ]
13. Muñoz N, Franceschi S, Bosetti C, Moreno V, Herrero R, Smith JS et al. Role of parity and human papillomavirus in cervical cancer: The IARC multicentric case-control study. Lancet 2002;359:1093-1101. [ Links ]
14. Moreno V, Bosch FX, Muñoz N, Meijer CJ, Shah KV, Walboomers JM et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: The IARC multicentric case-control study. Lancet 2002;359:1085-1092. [ Links ]
15. Plummer M, Herrero R, Franceschi S, Meijer CJLM, Snijders P, Bosch FX et al. Smoking and cervical cancer: Pooled analysis of are a multicentric case-control study. Cancer Causes R Control. In press. [ Links ]
16. Smith JS, Herrero R, Bosetti C, Muñoz N, Bosch FX, Eluf-Neto J et al. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. J Natl Cancer Inst. 2002;94:1604-1613. [ Links ]
17. Smith JS, Muñoz N, Bosetti C, Herrero R, Bosch FX, Eluf-Neto J et al. Chlamydia trachomatis as an HPV cofactor in the etiology of invasive cervical cancer: A pooled analysis of seven countries. 19th International Papillomavirus Conference; 2001;Florianopolis, Brazil. O-135. [ Links ]
18. Kols A, Sherris J. HPV Vaccines: Promise and challenges. Seattle (WA): PATH, 2000. [ Links ]
19. Harro CD, Pang YY, Roden RB, Hildesheim A, Wang Z, Reynolds MJ et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst 2001;21: 284-292. [ Links ]
20. Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomaviruses in invasive cervical cancer worldwide: A meta-analysis. J Natl Cancer Inst Br J. Cancer 2003;88:63-73. [ Links ]
21. Evans TG, Bonnez W, Rose RC, Koenig S, Demeter L, Suzich JA et al. A phase 1 study of a recombinant viruslike particle vaccine against human papillomavirus type 11 in healthy adult volunteers. J Infect Dis 2001;183:1485-1493. [ Links ]
22. Koutsky LA, Wheeler Cm, Brown DR, Barr E, Alvarez FB, Chiacchierini LM et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med 2002;347:1645-1651. [ Links ]
23. Franceshi S. Strategies to reduce the risk of virus-related cancers. Ann Oncol 2000;11:1091-1096. [ Links ]
24. International Agency for Research on Cancer. Monographs on the evaluation of carcinogenic risks to humans. Hepatitis viruses. IARC: Lyon, 1994; Vol. 59. [ Links ]
25. Gambia Hepatitis Study Group. The Gambia hepatitis intervention study. Cancer Res 1987;47:5782-5787. [ Links ]
26. Viviani S, Jack A, Hall AJ, Maine N, Mendy M, Montesano R et al. Hepatitis B vaccination in infancy in The Gambia: Protection against carriage at 9 years of age. Vaccine 1999;17:2946-2950. [ Links ]
27. Huang K, Lin S. Nationwide vaccination: A success story in Taiwan. Vaccine 2000;18 (Suppl 1):S35-S38. [ Links ]
28. Beral V, Hermon C, Muñoz N, Devesa SS. Cervical cancer. Cancer surv 1994;19/20:265-285. [ Links ]
29. Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J. Cancer incidence in five continents. International Agency for Research on Cancer, Lyon: Vol. VII; IARC Scientific publications No 143. 1997. [ Links ]
30. Konya J, Dillner J. Immunity to oncogenic human papillomaviruses. Adv Cancer Res 2001;82:205-238. [ Links ]
31. Nardelli-Haefliger D, Wirthner D, Schiller J, Lowy DR, Hildesheim A, Ponci F. Specific antibody levels at the cervix during menstrual cycle of women vaccinated with human papillomavirus 16 virus like particles. J Natl Cancer Inst 2003;95:1128-1137. [ Links ]
Address reprint requests:
Dr Martyn Plummer
150, Cours Albert Thomas
F-69372 Lyon, France
E-mail: plummer@iarc.fr
Received on: September 17, 2002
Accepted on: February 17, 2003














