Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Salud Pública de México
versión impresa ISSN 0036-3634
Salud pública Méx vol.45 supl.3 Cuernavaca ene. 2003
ARTICLE ARTÍCULOS
Cervical squamous and glandular intraepithelial neoplasia: Identification and current management approaches
Neoplasia intraepitelial cervical escamosa y glandular: identificación y estrategias de manejo
V Cecil Wright, MD
Professor Emeritus. Department of Obstetrics and Gynaecology. The University of Western Ontario. London, Ontario, Canada
ABSTRACT
Certain types of human papillomaviruses (HPV) are associated with squamous intraepithelial lesions and cancer and these are termed high-risk. HPV type 16 is detected in approximately half of the high-grade squamous intraepithelial lesions and cancer. Because of the high rate of spontaneous regression of low-grade squamous lesions, follow-up by cytology, colposcopy and possible biopsy appears preferable. Due to the higher rate of progression to malignancy of the high-grade lesions conservative treatment is recommended. One of the most common reasons for persistence relates to the human immunodeficiency virus. Adenocarcinoma in situ is an uncommon disorder and not well identified by cytologic sampling or colposcopic inspection. The diagnosis is made by cone biopsy, the specimen having negative margins for disease. Hysterectomy is the treatment procedure of choice unless fertility is an issue. Excisional methods (particularly electrosurgical loop) can interfere with accurate histological interpretation in some cases of both squamous disease and adenocarcinoma in situ. This paper is available too at: http://www.insp.mx/salud/index.html
Key words: squamous intraepithelial neoplasia; adenocarcinoma in situ; conservative treatment
RESUMEN
Ciertos tipos de virus del papiloma humano (VPH), denominados de alto riesgo, están asociados con lesiones escamosas intraepiteliales y cáncer invasor. El VPH tipo 16 es detectado en aproximadamente la mitad de las lesiones escamosas intraepiteliales de alto grado y cáncer. Sin embargo, existe una elevada proporción de regresión espontánea en lesiones escamosas de bajo grado, por lo que para su monitoreo es preferible la utilización de citología, colposcopía y biopsia. Asimismo, debido a la elevada tasa de progresión a malignidad de lesiones de alto grado se recomienda un tratamiento conservador. Una de las razones comunes relacionadas con la persistencia de infección por el VPH es el virus de inmunodeficiencia humana. Por otra parte, el adenocarcinoma in situ es un trastorno raro, no bien identificado en muestras citológicas o de inspección colposcópica; el diagnóstico se realiza mediante la biopsia de cono, y el espécimen debe tener márgenes negativos para enfermedad. La histerectomía es un tratamiento probable, a menos que la fertilidad esté siendo buscada. La escisión, particularmente por electrocirugía (loop), puede interferir con la interpretación histológica en algunos casos o de ambos, particularmente en enfermedades escamosas y adenocarcinoma in situ. En este artículo se describe ampliamente el manejo de la neoplasia intraepitelial escamosa y glandular. Este artículo también está disponible en: http://www.insp.mx/salud/index.html
Palabras clave: cáncer cervical; neoplasia intraepitelial escamosa; adenocarcinoma in situ; tratamiento conservador
Clinical and subclinical human papillomavirus (HPV) infections are among the most common sexually transmitted diseases today.1 Asymptomatic HPV infections can be detected in 5% to 40% of women of reproductive age.1 It is recognized that HPV infection is the central causal factor in cervical cancer.1,2 There are over 100 HPV types. At least 40 types infect the female and male anogenital tracts producing a spectrum of lesions ranging from genital condyloma to invasive cancer.1,3
Classification of HPV types
Based on their association with benign lesions, high-grade precursor lesions and invasive cancer, the anogenital HPV types can be classified into non-oncogenic and oncogenic risk categories. Low or non-oncogenic risk viruses include types 6,11,42,43,44 and they are associated with condylomata and some cases of low grade squamous intraepithelial lesions (CIN I) but rarely if ever invasive cancer. As a single oncogenic risk, type 16 is the most common one found in screening, high-grade (CIN II-III) squamous intraepithelial neoplasia and cancer.2,4,5 Using PCR-based tests, 99.7% of cervical cancers worldwide are positive for HPV.6 Viral types 16 and 18 predominate in glandular lesions (adenocarcinoma in situ and adenocarcinoma) with type 18 being the most common in adenocarcinoma and type 16 in adenocarcinoma in situ lesions.7,8
Squamous intraepithelial neoplasia
There is an increased incidence of cervical squamous intraepithelial lesions and a higher prevalence of oncogenic HPVs in human immunodeficiency virus (HIV) infected women because of CD4 T-cell suppression and viral load in both adolescents and adults 9-13 Persistent squamous intraepithelial lesions occur more often in HIV-infected women probably because of immunosuppression (CD 4 count < 200 cells/mm3).9-13
High risk type HPV infections of the cervix and vagina are transient in 80% of women. In these cases an intraepithelial lesion does not develop and the virus clears in 6 to 8 months.14,15 Intraepithelial neoplasia will develop in the other 20% of women but the high majority of these will similarly regress, the virus clears and the lesion subsequently disappears.4,14-16 Peak levels of high risk viral types (prevalence 20 to 25%) occur in women between 20 to 24 years of age.2,4 It is estimated that between 5% and 10% of low-grade squamous intraepithelial lesions progress to high-grade squamous intraepithelial lesions and rarely to cancer.17,18 In contrast 30% of untreated high-grade lesions of the cervix progress to invasive cancer.19 The progression of a low-grade lesion is thought to be due to the presence of an oncogenic viral type –specifically types 16 and 18. This suggests that HPV typing may be useful in predicting the likelihood of progression and the necessity of treating.20,21 Because few low-grade intraepithelial lesions (cervical intraepithelial neoplasia grade 1/CIN I) progress, many clinicians choose not to treat them but only to follow patients with cytology, colposcopy and at times biopsy. Any advancement in lesion grade requires treatment. Such candidates are usually young and nulliparous. CO2 laser ablation and cryosurgery are excellent options with cure rates exceeding 90%.22 Electrosurgical loop excision is another option. Since high-grade squamous intraepithelial lesions(including moderate dysplasia/CIN II and severe dysplasia/carcinoma in situ/CIN III) can progress to malignancy, treatment is recommended.
What is the best method of treatment?
Currently many investigators advocate electrosurgical loop excision for ectocervical high-grade squamous intraepithelial lesions.23-25 This is a retreat from the widespread practice in the last 20 years of ablation by cryosurgery, carbon dioxide laser vaporization, electrodiathermy and cold-coagulation. Most are office procedures and some require brief anesthesia. All ablative procedures rely on adequate colposcopic evaluation and correlation to assure that invasive cancer is not missed. Patients are cured using these techniques in 98% of cases with one or two procedures.22,26-30
The purported advantage of routine excision of colposcopically defined high-grade squamous intraepithelial neoplasia is that a specimen (or specimens) is always available. However, the following questions apply: a) is the specimen(s) always interpretable?; b) when interpretable is cancer ever missed?; c) do clear margins insure that no high-grade lesion or cancer is present?, and d) does the thermal effect interfere with interpretation of clear margins or with a diagnosis of adenocarcinoma?
At colposcopic examination one determines whether the examination is satisfactory or not and formulates a colposcopic impression. After the correlation process has been completed, the colposcopist must determine what the patient needs next. Is it: a) referral to a gynecologic oncologist for staging; b) diagnostic excision (conization); c) conservative local treatment, or d) follow-up?
The indications for ablative methods or loop excision are: a) cytology, colposcopy, and pathology must correlate to establish an accurate tissue diagnosis; b) the entire transformation zone must be colposcopically defined; c) the colposcopist must be certain from the qualitative assessment of the transformation zone that no invasive cancer is present; d) the intraepithelial lesion must occupy the ectocervix with no extension into the endocervical canal, and e) preferably the patient is not pregnant.30
In contrast excisional procedures are required when: a) discrepancies exist between cytology, colposcopy and histology; b) significant lesions are located in the endocervical canal and require tissue for histological sampling; c) cytology or colposcopy suggests possible invasive carcinoma that has not been proven by colposcopically directed biopsy; d) colposcopic biopsy indicates microinvasive squamous disease or adenocarcinoma in situ, or e) when colposcopy is unsatisfactory.30 In these cases loop electrosurgery is not considered appropriate because the apical margin might be positive and electric current can interfere with histological interpretation. Laser excision or electrosurgical needle excision, removing a cylindrical specimen, with the apex of the specimen being cut with a scalpel or scissors (creating no thermal effect) produces a good quality specimen in the author's experience.30 However, in these situations, some pathologists prefer the use of the scalpel and doing a cold knife conization.31
In managing cervical intraepithelial lesions, regardless of method, the following must be taken into consideration in designing the excision parameters: a) intraepithelial neoplasia (particularly CIN III /severe dysplasia/carcinoma in situ) can extend into cervical crypts up to 5.2mm32,33 –it has been postulated that destruction/excision of lesions to a depth of 3.8 mm would eradicate all involved crypts in 99.7% of patients;32 b) the radial linear length of squamous intraepithelial neoplasia varies between 2 and 22 mm (Figure 1),33,34 and c) invasive cancer typically occurs on the canal side– that is, the worst pathology is located centrally.35,36 Therefore, accepting the known dimensions, to remove the likely diseased tissue with the least volume of normal tissue, one can conceptualize the three-dimensional geometry of disease in any given patient depending on the location of the squamocolumnar junction. Then appropriate operative techniques can be applied to remove or destroy it (Figure 2).37 This is the basis of ablating or removing diseased tissue in a cylindrical manner or combining excision with ablation (the combination procedure) whether using central excision plus peripheral vaporization or electrosurgery with a combination of loop excisions or using the electrosurgical needle (Figures 3 and 4).
Treatment, especially by excisional methods, can cause symptomatic stenosis with canal narrowing, although this is an infrequent complication. The most common patient symptom is severe dysmenorrhea. Narrowing can lead to difficulty in obtaining satisfactory cytology.38,39 Stenosis is related to hypoestrogenic states: a) women of reproductive age with oligomenorrhea or amenorrhea; b) women on a low-dosage contraceptive pill who have oligomenorrhea or amenorrhea; c) women who are post-partum and lactating; d) post-menopausal women who are not on hormone replacement therapy with amenorrhea; e) women who are on estrogen and progesterone therapy with amenorrhea, and f) medroxyprogesterone acetate patients.39 The incidence of cervical stenosis can be reduced by the use of cyclic conjugated estrogen and progesterone to induce menses before and after treatment for several months.39 Long length (height) of the specimen (>20mm) and an entirely endocervical lesion appear to be independent risk factors for stenosis.38 The majority of cervical stenosis cases (95%) are diagnosed at the first post-operative assessment and are usually easily treated by dilation of the endocervical canal.38
When thermal energy is used, heat may interfere with accurate histological interpretation. Some investigators found loop specimens in general difficult to interpret due to the thermal artifact and fragmentation. This interfered with margin assessment and grading of the lesion.40-42 In contrast, others experienced no difficulties in specimen assessment.43,44 Furthermore, unrecognized malignancy has been identified in hysterectomy specimens (a surprise finding) following loop excision.45
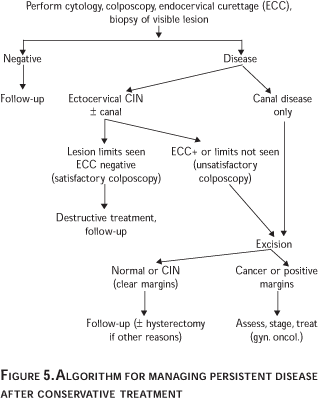
Regardless of the method of treatment persistent disease occurs. After ablative methods it is usually identified at the first visit by colposcopy using acetic acid and iodine staining and including cytology.30 No earlier than three months after treatment the patient is submitted to the standard colposcopic examination with biopsies (including endocervical curettage) if warranted. If significant disease is identified, retreatment is usually necessary. The technique depends upon disease location. For excisional procedures, it is important to know whether there was an endocervical (apical) margin positive or an ectocervical margin positive. This is because a positive endocervical margin with CIN III disease can signal potential remaining cancer in 3 to 8% in the intact cervix (potentially, the worst disease occurs centrally).46-50 In excisional procedures the reporting of negative margins is associated with a 90 to 97% normal follow-up51 versus a 60 to 65% persistent disease rate with positive margins and positive endocervical curettage (ECC) –the ECC being done immediately after the conization.51,52 That is, a histological report of incomplete excision of cervical dysplasia does not necessarily equate with residual disease. Others have found abnormal cytology to be an excellent predictor of residual disease.52 Figure 5 illustrates an algorithm to investigate persistent disease after conservative management. Despite negative margins and normal follow-up there is a small lifetime risk of developing an invasive cancer in the intact cervix of 2 to 4%.53,54
In some cases simple hysterectomy for the high-grade squamous case may still be required for the following reasons: a) failed conservative management; b) extensive disease involving the cervix and vagina where conservative management may be difficult; c) the presence of co-existing pathology (not resolved conservatively) such as large fibroids, uterine prolapse, endometriosis or intractable menorrhagia; d) technical difficulties in exposure; e) unresolved post-conservative treatment stenosis; f) definitive management of adenocarcinoma in situ; g) to control hemorrhage post-excision (conization), and h) cancer phobia.54 If a hysterectomy is done for some reason, invasive cancer still develops in the vaginal vault in 0.1 to 2.0% of cases.53-56
Cervical adenocarcinoma in situ
Adenocarcinoma in situ (AIS) of the uterine cervix was first described in 1953 by Freidel and McKay.57 Glandular lesions have a morphological spectrum (similar to squamous) ranging from mild changes to severe abnormalities termed cervical intraepithelial glandular neoplasia and abbreviated as CIGN, CGIN or GIN. This spectrum has been divided into low grade and high grade CIGN.58,59 In this article the specific entity AIS (high grade CIGN) will be discussed.
Adenocarcinoma in situ is uncommon. The ratio of AIS to CIN III lesions (severe dysplasia/carcinoma) is 1:50 or 2%.60-62 The majority of AIS cases (46 to 72%) contain a counterpart squamous component –termed "mixed disease"– which is usually a CIN III lesion.61-63 Furthermore, AIS is less commonly diagnosed than its malignant counterpart, the latter accounting for 6 to 18% of all invasive cancers.61,64,65 The usual age range of women with AIS is from 29 to 46 years with an average age of 35.8 years. 59,65
Making the diagnosis of AIS by the clinician is challenging. The lesion is frequently overlooked because of the absence of clinical findings, the presence of normal cytology or cytology reflecting squamous disease and unfamiliar colposcopic patterns.37,66,67 Cullimore et al noted that women with pure AIS were 4.8 years older than women with mixed disease. This finding suggests that cytologic studies are more accurate in identifying the squamous component than the glandular component.59 Only recently have authors drawn attention to the colposcopic features suggestive of AIS.68-70 The relative infrequency of AIS cases as compared to squamous disease has not permitted most physicians to accumulate large series or gain experience with colposcopic identification.
Basically there are three colposcopic presentations of AIS lesions. The most common colposcopic appearance is a papillary expression resembling an immature transformation zone (Figure 6). Second is a flat, variegated red and white area resembling an immature transformation zone (Figure 7). The least common is one or more isolated, elevated, individual, densely acetowhite lesions overlying columnar epithelium.68-70 When glandular and squamous lesions coexist, the squamous component is more likely to be noted because it is more likely visible. In mixed disease, the glandular lesion can abut the glandular lesion, be sandwiched between two squamous lesions or lie above the squamous lesion (Figure 8).68,69 There is no single colposcopic appearance which characterizes adenocarcinoma in situ. In many cases colposcopic appearances of AIS mimic other conditions (Table I).
AIS lesions can be small, focal and easily missed.17,59 Ectocervical expression occurs in 53% of cases, the endocervical canal in 5% of cases and contiguous involvement in 38%, indicating that 95% of AIS cases are available for partial or complete colposcopic scrutiny.71 Forty-eight percent of lesions involve one quadrant whereas only 10% occupy all four.71
Measurements (linear length) of AIS lesions (the distance over the tissue surface between caudal and cephalad edges) have been studied. The distance usually does not exceed 15 mm and rarely does it encompass the entire glandular epithelium.17,72,73 Younger women, particularly those less than age 36 years, have a significant reduction in the proximal extent of AIS. Nicklin et al found that women younger than age 36 years have a mean lesion length of 5.6 mm, versus 10.8 mm for women age 36 years and older (a statistically significant difference).72 Only 1 of 14 younger women from a series of 31 patients, had a linear length of more than 10 mm. In contrast 9 of 17 women in the older group had lesion lengths of more than 10 mm, with a maximum of 25 mm.72 Bertrand et al studied the highest focus of cervical involvement of AIS measured from the maximal convexity of the cervix in hysterectomy specimens. The highest focus did not exceed 19.9 mm in 78.9% of cases, the highest focus being 29.9 mm.74 Such measurements however do not reflect the true linear length of disease but rather provide guidelines for designing cylindrical excisional specimen measurements to account for the distribution particularly for endocervical canal involvement.
As in squamous intraepithelial neoplasia, AIS can extend into underlying cervical crypts. Involvement averages 2.5 mm and extension is usually no more than 4 mm, maximum 6 mm.37,63,72,73 Also younger women (age 35 or less) had a lesser depth of disease than did older women.72
Multifocal lesions (so called "skip lesions") represent foci involving different portions of the endocervical mucosa. By definition, this is when a normal radial histological section separates two areas of AIS.74 Such lesions are uncommon and occur in 6.5 to 15% of AIS lesions.17,74 Multifocality of AIS does not appear to correlate with a higher incidence of residual disease.31
AIS can involve both superficial and deep crypts that are covered by metaplastic or dysplastic squamous epithelium. This is termed "buried disease" and occurs in 60% of cases.63,74 Although the crypts open through such tissue, the glandular component will not be colposcopically visible.
AIS lesions are to be suspected when any of the following colposcopic findings are observed: a) a lesion overlying columnar epithelium and not contiguous with the squamocolumnar junction; b) large gland openings (Figure 7); c) papillary-like lesions (Figure 6); d) epithelial budding; e) variegated red and white lesions (Figure 7), and f) atypical angioarchitecture such as waste-thread-like, tendril-like, root-like (Figure 8) or character-writing-like vessels and single and multiple-dot-like formations (Figure 6), the latter seen in the tips of the papillary excrescences. The differential of similar appearing lesions is shown in Table I.68
Having suspected the glandular lesion, the clinician should consider the following in AIS management: a) patient's age; b) lesion location (ectocervical, endocervical, or both – colposcopic assessment can be helpful); c) three dimensional lesion geometry (linear length and crypt involvement); d) potential for buried disease; e) mixed disease (presence of a squamous component); f) specimen margin status post-excision; g) patient's desire for fertility, and h) patient's compliance.
When AIS is found on biopsy or suspected cytologically or colposcopically, an excisional procedure is required, producing a specimen with negative margins (ie, free of disease) – the latter to help ensure that no adenocarcinoma is present. The excisional procedure should attempt to account for the distribution of disease (linear length, crypt involvement, and disease location). As in squamous intraepithelial neoplasia, the configuration (base and height dimensions) will vary. A cylindrical shaped specimen best accounts for disease distribution (Figure 9).63,74,75 It is recommended that the procedure be done under colposcopic guidance noting the lower lesion border (which is usually at or very near the squamocolumnar junction), and if possible, noting or estimating the upper margin, that is, the entire potential linear length.63,74,75 These parameters serve as a guide for determining the dimensions of the cylindrical specimen.
The preferred instrument for the excisional procedure to provide the best interpretable specimen is a subject of debate. From the pathologist's standpoint, the cold knife conization is the safest and best therapeutic modality, provided that the cone specimen is adequately sampled and the margins are free.37,76 Other investigators have found that the use of laser or electrosurgical needle to create the deep margin plus scalpel excision of the cylinder's apex (producing a non-thermal effect) to be satisfactory.75 It must be recognized that a cold knife conization can be a formidable operation. There is general consensus that electrosurgical loop may not be the procedure of choice. This is because electric current follows the path of least resistance (into crypt mucus) and thus it can potentially distort the glandular epithelium (causing pseudostratification or streaming of nuclei parallel to the electric field) making it difficult to histologically differentiate between in situ and invasive disease.37,77 Furthermore such loop specimens are often fragmented due to several passes making evaluation of the margins difficult.37 Others have found that the use of loop excision in the management of AIS is acceptable when there is a single specimen with clear margins.78
Cumulative studies show that positive margins are of significance due to persistent AIS in 12.5 to 80% of cases.59,60,71,72,79-86 Adenocarcinoma is found in 12.5 to 50% of such cases.71,80,82 Other studies did not identify malignancy (adenocarcinoma) in cases with positive margins on follow-up histology.17, 59, 71-73,81,83,84 In positive margin cases, repeat excision is recommended to obtain negative margins in the conservatively managed patient who desires future childbearing. Repeat excision, producing negative margins is also recommended before a simple hysterectomy as definitive treatment. Failure to do so may result in inappropriate surgery (simple hysterectomy instead of radical hysterectomy), should adenocarcinoma be found in the extirpated uterine cervix.75
Studies indicate that if excised specimens have negative margins then conservative management is possible in those women who desire future child bearing.59,71,73,82,86 However, negative margins are associated with persistent AIS in 8.3 to 50% of cases.71,80-86 These findings suggest that cervical conization should not necessarily be considered a definitive treatment for AIS in the presence of negative margins.30 Studies have rarely identified adenocarcinoma even when specimens had negative margins.79,80,87
For the conservatively managed patient, follow-up management should consist of cytology, colposcopy, and endocervical curettage every four months for one year and every six months thereafter.80,87 Patients who choose to be followed conservatively must be counseled about the importance of compliance and the potential risks of undetected and recurrent glandular disease, despite negative follow-up findings.79,81
References
1. Franco EL, Durante-Franco E, Ferenczy A. Cervical cancer: Epidemiology, prevention, and the role of the human papillomavirus infection. Can Med Assn J 2001;164:1017-1025. [ Links ]
2. Sellors JW, Loring AT, Mahony JB, Mielzynska I, Lytwyn A, Roth P et al. Comparison of self-collected vaginal, vulvar and urine samples for human papillomavirus testing to detect high-grade squamous intraepithelial lesions. Can Med Assn J 2000;163:513-518. [ Links ]
3. Bristow RE, Montz FJ. Human papillomavirus: Molecular biology screening applications in cervical dysplasia – A primer for primary care physicians. Prim Care Update Ob/Gyn 1998;5:238-246. [ Links ]
4. Ho GY, Burk RD, Klein S, Kadish AS, Chang CJ, Palan P et al. Persistent genital human papillomavirus infection as a risk factor for persistent dysplasia. J Natl Cancer Inst 1995;87:1365-1371. [ Links ]
5. Nagai Y, Maehamat T, Asato T, Kanazawa K. Persistence of human papillomavirus infections after therapeutic conization for CIN III. Is it an alarm for recurrence? Gynecol Oncol 2000;79:294-299. [ Links ]
6. Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer A, Shah KV et al. Human papillomavirus is a necessary cause of invasive cancer worldwide. J Pathol 1999;189:12-19. [ Links ]
7. Andersson S, Rylander E, Larsson B, Strand A, Silfersvard C, Wilander E. The role of human papillomavirus in cervical adenocarcinoma carcinogenesis. Eur J Cancer 2001;37:246-250. [ Links ]
8. Riethdorf S, Riethdorf L, Milde-Langosch K, Park JW, Loning T. Differences in HPV 16 and HPV 18 E6/E7 oncogenic expression between in situ and invasive adenocarcinoma of the cervix uteri. Virchows Arch 2000; 437:491-500. [ Links ]
9. United States Public Health Service/Infectious Diseases Society of America. Guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. Ann Intern Med 1997;127:922-946. [ Links ]
10. Ellerbrock TV, Chiasson MA, Bush TJ, Sun XW, Sawo D, Brundley K et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA 2000;283:1031-1037. [ Links ]
11. Sun XW, Kuhn L, Ellerbrook TV, Lungu O, Chiasson MA, Bush TJ et al. Human papillomavirus infection in women infected with the human immunodeficiency virus. N Engl J Med 1997;337:1343-1349. [ Links ]
12. Heard I, Jean-Michel T, Schmitz V, Mandelbrot L, Kazatchkine MD, Orth G. Increased risk of cervical disease among immunodeficiency. Virus-infected women with severe immunosuppression and human papillomavirus load. Obstet Gynecol 2000;96:403-409. [ Links ]
13. Moscicki AB, Ellenberg JH, Vermund SH, Holland CA, Darrah T, Crowley-Nowick PA et al. Prevalence of and risks for cervical human papillomavirus infection and squamous intraepithelial lesions in adolescent girls: Impact of infection with human immunodeficiency virus. Arch Pediatr Adolesc Med 2000;154:127-134. [ Links ]
14. Rozendaal L, Walboomers JMM, van der Linden JC, Voorhorst FJ, Kenemans P, Helmerhorot TJM et al. PCR-based high risk HPV test in cervical cancer screening gives objective risk assessment of women with cytomorophologically normal cervical smears. Int J Cancer 1996;68:766-769. [ Links ]
15. Nobbenhuis MAE, Walboomers JMM, Helmerhorst TJM, Tozendaal L, Remmink AJ, Risse AKT et al. Relation of human papillomavirus status to cervical lesions and consequences for cervical-cancer screening: A prospective study. Lancet 1999;354:20-25. [ Links ]
16. Meijer CJLM, Snijders PJF, Van den Brule AJC. Screening for cervical cancer: Should we test for infection with high risk HPV? Can Med Assn J 2000;163:535-538. [ Links ]
17. Östör AG. Natural history of cervical intraepithelial neoplasia: A critical review. Int J Gynecol Oncol 1993;12:186-192. [ Links ]
18. Naisell K, Roger V, Naisell M. Behavior of mild dysplasia during long term follow-up. Obstet Gynecol 1986;67:665-669. [ Links ]
19. McIndole WA, McLean MR, Jones RW, Mullins PR. The invasive potential of carcinoma in situ of the cervix. Obstet Gynecol 1984;64:451-458. [ Links ]
20. Campion MJ, McCane DJ, Cuzick J, Singer A. Progressive potential of mild cervical atypia, prospective cytologic, colposcopic and virologic study. Lancet 1986;2:237-240. [ Links ]
21. Schneider A, Meihardt G, Devilliers EM, Gisssman L. Sensitivity of the cytologic diagnosis of cervical condyloma in comparison with HPV DNA hybridization studies. Acta Cytopathol 1987;3:250-255. [ Links ]
22. Persad VL, Pierotic MA, Guijon FB. Management of cervical neoplasia: A 13 year experience. J Lower Gen Tract Dis 2001;5:199-203. [ Links ]
23. Mor-Yosef S, Lopes A, Pearson S, Monghan JM. Loop diathermy cone biopsies. Obstet Gynecol 1990;75:884-886. [ Links ]
24. Prendiville W, Cullimore J, Norman S. Large loop excision of the transformation zone (LLETZ): A new method of management for women with cervical intraepithelial neoplasia. Br J Obstet Gynaecol 1989;96:1054-1060. [ Links ]
25. Ferenczy A, Chouroun D, Arseneau J. Loop electrosurgical excision procedure for squamous intraepithelial lesions of the cervix: Advantages and potential pitfalls. Obstet Gynecol 1996;87:332-337. [ Links ]
26. Wright VC, Davies E, Riopelle MA. Laser surgery for cervical intraepithelial neoplasia: Principles and results. Am J Obstet Gynecol 1983;145: 181-185. [ Links ]
27. Duncan ID. The Semm cold coagulator in the management of cervical intraepithelial neoplasia. Clin Obstet Gynaecol 1983;26: 996-999. [ Links ]
28. Chanen W, Rome RM. Electrocoagulation diathermy in the treatment of cervical dysplasia and carcinoma in situ: A 15 year survey. Obstet Gynecol 1983;61:673-677. [ Links ]
29. Bryson SC, Lenehan P, Lickrish GM. The treatment of grade 3 cervical intraepithelial neoplasia with cryotherapy: An 11 year experience. Am J Obstet Gynecol 1985;151:201-205. [ Links ]
30. Wright VC. Carbon dioxide laser surgery for the cervix and vagina: Indications, complications and results. Comp Ther 1988;14:54-64. [ Links ]
31. Östör AG, Duncan A, Quinn M, Rome R. Adenocarcinoma in situ of the uterine cervix: An experience with 100 cases. Gynecol Oncol 2000;79: 207-210. [ Links ]
32. Anderson MC, Hartley RB. Cervical crypt involvement by intraepithelial neoplasia. Am J Obstet Gynecol 1980;55:546-549. [ Links ]
33. Boonstra H, Aalders JG, Koudstaal J, Oosrerhuis JW, Janssens J. Minimum extension and appropriate topographic position of tissue destruction for treatment of cervical intraepithelial neoplasia. Obstet Gynecol 1990;75:227-231. [ Links ]
34. Przybora LA, Plutowa A. Histologic topography of carcinoma in situ of the cervix uteri. Cancer 1959;12:268-273. [ Links ]
35. Carron RP, Gall EA. Preinvasive carcinoma and precancer metaplasia of the cervix. Am J Pathol 1954;30:15-19. [ Links ]
36. Scott RB, Reagan JW. Diagnostic cervical biopsy technique for the study of early cancer: Value of the cold-knife conization procedure. JAMA 1956;343-348. [ Links ]
37. Wright VC, Riopelle MA. The geometry of cervical intraepithelial neoplasia as a guide to its eradication. Cervix 1986;4:21-38. [ Links ]
38. Baldauff JJ, Dreyfus M, Ritter J, Meyer P, Philippe E. Risk of cervical stenosis after large loop or laser conization. Obstet Gynecol 1996;88: 933-938. [ Links ]
39. Lickrish GM. Colposcopy in the management of cervical intraepithelial neoplasia: Problems and suggestions. J Soc Obstet Gynaecol Can 2000; 22:429-434. [ Links ]
40. Montz FJ, Holschnidrer CH, Thompson CDR. Large loop excision of the transformation zone: Effect on pathologic interpretation of resection margins. Obstet Gynecol 1993;81:976-982. [ Links ]
41. Ioffe OB, Brooks SE, De Rezende RB, Silverberg SG. Artifact in cervical LLETZ specimens: Correlated with follow-up. Int J Gynaecol Pathol 1999;18:115-121. [ Links ]
42. Dalrymple C, Russel P. Thermal artifact after diathermy loop excision and laser cone biopsy. Int J Gynaecol Cancer 1999;9:238-242. [ Links ]
43. Baggish MS, Barash F, Noel Y, Brooks M. Comparison of thermal injury zones in loop electrical and laser cervical excisional conization. Am J Obstet Gynecol 1992;166:545-548. [ Links ]
44. Spitzer M, Chernys AE, Seltzer VL. The use of large loop excision of the transformation zone in an inner city population. Obstet Gynecol 1993;82:731-735. [ Links ]
45. Whitely PE, Olah KS. Treatment of cervical intraepithelial neoplasia: Experience with low-voltage diathermy loop. Am J Obstet Gynaecol 1990; 162:1272-1277. [ Links ]
46. Grundsell H, Alm P, Larsson G. Cure rates after conization for early cervical neoplasia. Ann Chir Gynecol 1993;72:218-222. [ Links ]
47. Mohamed-Noor K, Quinn MA, Tan J. Outcomes after cervical cold knife conization with complete and incomplete excision of abnormal epithelium: A review of 699 cases. Gynecol Oncol 1997;67:34-38. [ Links ]
48. Ostegard DR. Prediction of cervical clearance of cervical intraepithelial neoplasia by conization. Obstet Gynecol 1980;56:77-80. [ Links ]
49. Killackney MA, Jones WB, Lewis J, Jr. Diagnostic conization of the cervix: Review of 460 cases. Obstet Gynecol 1986;67:766-770. [ Links ]
50. Benedet JL, Anderson GH, Boyes DA. Colposcopic accuracy in the diagnosis of microinvasive and occult invasive carcinoma of the cervix. Obstet Gynecol 1985;65:551-561. [ Links ]
51. Schermerhorn TJ, Hodge J, Saztman AK, Hackett TE, Sprance HE, Harrison TA. Clinicopathologic variables predictive of residual dysplasia after cervical conization. J Reprod Med 1997;42:189-192. [ Links ]
52. Buxton EJ, Luesley DM, Wade-Evans T, Jordan JA. Residual disease after cone biopsy: Completeness of excision and follow-up cytology as predictive factors. Obstet Gynecol 1987;70:529-532. [ Links ]
53. Kolstad P, Valborg K. Long term follow-up of 1121 cases of carcinoma in situ. Obstet Gynecol 1976;48:125-129. [ Links ]
54. Jordan JA. Symposium on cervical dysplasia. I. Excisional methods. Colpos Gynecol Laser Surg 1984;4:271-274. [ Links ]
55. Creasman WT, Parkin RT. Management of early cervical neoplasia. Clin Obstet Gynecol 1975; 18:233-238. [ Links ]
56. Boyes DA, Worth J, Fidler HK. The results of 4389 cases of preinvasive cervical squamous carcinoma. J Obstet Gynaecol Br Commwlth 1979; 77:769-773. [ Links ]
57. Friedell GH, McKay DG. Adenocarcinoma in situ of the endocervix. Cancer 1953;6:887-897. [ Links ]
58. Gloor E, Hurlimann J. Cervical intraepithelial glandular neoplasia (adenocarcinoma in situ and glandular dysplasia). Cancer 1986;58: 1272-1282. [ Links ]
59. Cullimore JE, Luesley DM, Rollason TP, Byrne P, Buckley CH, Williams DR et al. A prospective study of conization of the cervix in the management of cervical intraepithelial glandular neoplasia (CIGN) - A preliminary report. Br J Obstet Gynaecol 1992;99:314-318. [ Links ]
60. Anderson MC. Glandular lesions of the cervix: Diagnostic and therapeutic dilemmas. Baillières Clin Obstet Gynaecol 1995;9:105-119. [ Links ]
61. Christopherson WM, Nealson N, Gray LA. Noninvasive precursor lesions of adenocarcinoma and mixed adenosquamous carcinoma of the cervix uteri. Cancer 1981;48:768-773. [ Links ]
62. Boon ME, Baak JPA, Kurver PJH. Adenocarcinoma in situ of the cervix. Cancer 1981;48:768-773. [ Links ]
63. Colgan TJ, Lickrish GM. The topography and invasive potential of cervical adenocarcinoma in situ, with or without associated dysplasia. Gynecol Oncol 1990;36:246-249. [ Links ]
64. Liu S, Semenci R, Mao Y. Cervical cancer: The increasing risk of adenocarcinoma and adenosquamous carcinoma in younger women. Can Med Assn J 2001;164:1152-1154. [ Links ]
65. Plaxe SC, Saltzstein SL. Estimation of the duration of the preclinical phase of cervical adenocarcinoma suggests there is ample opportunity for screening. Gynecol Oncol 1999;75:55-61. [ Links ]
66. Laverty CR, Farnsworth A, Thurlse J, Bowditch R. The reliability of a cytological prediction of cervical adenocarcinoma in situ. Aust NZ J Obstet Gynaecol 1988;28:307-312. [ Links ]
67. Mitchell H, Medley G, Gordon I, Giles G. Cervical cytology reported as negative and risk of adenocarcinoma of the cervix: No strong evidence of benefit. Br J Cancer 1995;71:894-897. [ Links ]
68. Wright VC, Shier RM. Colposcopy of adenocarcinoma in situ and adenocarcinoma – Differentiation from Other Cervical Lesions. Houston: Biomedical Communications, 2000. [ Links ]
69. Wright VC. Colposcopic features of cervical adenocarcinoma in situ and adenocarcinoma and management of preinvasive disease. En: Apgar B, Bronztman GL, Spitzer M. Colposcopy – Principles and practice. Philadelphia: WB Saunders, 2001:301-320. [ Links ]
70. Coppleson M, Atkinson KH, Dalrymple JC. Cervical squamous and glandular neoplasia: Clinical features and review of management. En: Coppleson M. Gynecologic Oncology. Edinburgh: Churchill Livingston, 1992: 571-607. [ Links ]
71. Muntz HG, Bell DA, Lage JM, Geoff BA, Feldman S, Rice LW. Adenocarcinoma in situ of the uterine cervix. Obstet Gynecol 1992;80:935-939. [ Links ]
72. Nicklin JL, Wright RG, Bell JR, Samaratunga H, Cox NC, Ward BG. A clinicopathological study of adenocarcinoma in situ of the cervix. The influence of cervical HPV infection and others factors, and the role of conservative surgery. Aust NZ J Obstet Gynaecol 1991;2:179-183. [ Links ]
73. Andersen ES, Arffman E. Adenocarcinoma in situ of the uterine cervix: A clinicopathologic study of 36 cases. Gynecol Oncol 1989; 35:1-6. [ Links ]
74. Bertrand M, Lickrish GM, Colgan TJ. The anatomical distribution of cervical adenocarcinoma in situ. Am J Obstet Gynecol 1987;1:21-26. [ Links ]
75. Wright VC, Dubuc-Lissoir J, Ehlen T, Heywood M, Plante M. Guidelines on adenocarcinoma in situ of the cervix: Clinical features and review of management. J Soc Obstet Gynaecol Can 1999;77:699-706. [ Links ]
76. Wright TC, Jr., Cox TJ, Massad LS, Twiggs LB, Wilkinson EJ. 2001 consensus guidelines for management of women with cervical cytological abnormalities. J Lower Gen Tract Dis 2002;6:127-143. [ Links ]
77. Thomas PA, Zalaski MS, Ohlhausen WW, Raab SS, Brenda JJ. Cytomorphologic characteristics of thermal injury related to endocervical brushing following loop electrosurgical excision procedure. Diagn Cytopathol 1996; 14:212-215. [ Links ]
78. Houghton SJ, Shafi MI, Rollason TP, Luesley DM. Is loop excision adequate primary treatment of adenocarcinoma in situ of the cervix? Br J Obstet Gynaecol 1997;104:325-329. [ Links ]
79. Poynor EA, Barakat RR, Hoskins WJ. Management follow-up of patients with adenocarcinoma in situ of the uterine cervix. Gynecol Oncol 1995;57:158-164. [ Links ]
80. Widrich T, Kennedy AW, Myers TM, Hart WR, Wirth S. Adenocarcinoma in situ of the uterine cervix: Management and outcome. Gynecol Oncol 1996;61:304-308. [ Links ]
81. Wolf JK, Levenback C, Malpica A, Morris M, Burke T, Follen Mitchell M. Adenocarcinoma in situ of the cervix: Significance of cone biopsy margins. Obstet Gynecol 1996;88:82-86. [ Links ]
82. Denehy TR, Gregori CA, Breen JL. Endocervical curettage, cone margins and residual adenocarcinoma in situ of the cervix. Obstet Gynecol 1997;90:1-6. [ Links ]
83. Im DO, Duska LR, Rosenshein NB. Adequacy of conization margins in adenocarcinoma in situ of the cervix as a predictor of residual disease. Gynecol Oncol 1995;59:179-182. [ Links ]
84. Luesley DM, Jordan JA, Woodman CBJ, Watson N, Williams DR, Waddell C. A retrospective review of adenocarcinoma-in-situ and glandular atypia of the uterine cervix. Br J Obstet Gynaecol 1987;94:699-703. [ Links ]
85. Hopkins MP, Roberts JA, Schmidt RW. Cervical adenocarcinoma in situ. Obstet Gynecol 1988;71:842-844. [ Links ]
86. Weisbrot IM, Stabinsky C, Davis AM. Adenocarcinoma in situ of the uterine cervix. Cancer 1972;29:1179-1187. [ Links ]
87. Kennedy AW, Tabbakh GH, Biscotti CV, Wirth S. Invasive adenocarcinoma of the cervix following LLETZ (Large Loop Excision of the Transformation Zone). Gynecol Oncol 1995;58:274:277. [ Links ]
Address reprint requests to:
Dr. V. Cecil Wright
10829 Old River Road
Komoka, ON, NOL 1RO Canada
E-mail: vcwright@biomedical-communications.com
Received on: February 27, 2003
Accepted on: March 5, 2003














