Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Salud Pública de México
versión impresa ISSN 0036-3634
Salud pública Méx vol.43 no.6 Cuernavaca nov./dic. 2001
Evaluation of genotoxic activity of maleic hydrazide, ethyl methane sulfonate, and N-nitroso diethylamine in Tradescantia
Carlos Alvarez-Moya, Dr en C,(1) Anne Santerre-Lucas, Dr en C,(1) Guillermo Zúñiga-González, Dr en C,(2) Olivia Torres-Bugarín, Dr en C,(3) Eduardo Padilla-Camberos, Biól,(4) Alfredo Feria-Velasco, Dr en C.(4)
Alvarez-Moya C, Santerre-Lucas A, Zúñiga-González G, Torres-Bugarín O, Padilla-Camberos E, Feria-Velasco A.
Evaluation of genotoxic activity of maleic hydrazide, ethyl methane sulfonate, and N-nitroso diethylamine in Tradescantia.
Salud Publica Mex 2001;43:563-569.
The English version of this paper is available too at: http://www.insp.mx/salud/index.html
Abstract
Objective. To assess the genotoxic activity of N-nitroso diethylamine (NDEA), maleic hydrazide (MH), and ethyl methane sulfonate (EMS) using two systems: the comet assay on nuclei from Tradescantia, and the pink mutation test on Tradescantia staminal hairs (clone 4430). Material and Methods. Tradescantia cups was obtained from Laboratorio de Citogenética y Mutagénesis del Centro de Ciencias de la Atmósfera de la Universidad Nacional Autónoma de México and treated with: N-nitroso diethylamine (NDEA) at 1, 5, 10 mM, maleic hidrazide (MH) at 1, 5, 10 mM and ethyl methane sulfonate (EMS) at 15, 30 and 45 mM; and used in both pink mutation assay and comet assay using cellular nuclei from Tradescantia staminal hairs. The observation of staminal hair was realized along eight days (6-14) after treatment), flowers produced day 14 after treatment were utilized done according to Underbrink. In previous reports on plants, were comet assay was used, breaking cellular wall and separating by centrifugation gradient are necessary. Here, nuclei from staminal hairs were obtained by squashing the cells (is not necessary to utilize to break special procedure cellular wall), collected using a nylon mesh of 80Mm and next the comet assay was applied. Student's T test was the statistical test used for analyzing the comet assay data. Results. Both assays showed a great sensitivity to the studied mutagens. A relationship between the dose-pink event and the dose-tail length was evident. Even though the Tradescantia mutation assay is a sensitive test with MH and EMS, low doses of NDEA were not able to induce a significant increase in the pink event frequencies; however, the comet assay was able to detect the mutagenic effect of NDEA at the same dose. Thus, it is clear that the comet assay is highly sensitive to the lowest dose of chemical mutagens. Conclusions. The comet assay on nuclei from Tradescantia staminal hairs is a useful tool to monitor genotoxic agents; it is simple, highly sensitive, and faster than the pink mutation test. The English version of this paper is available too at: http://www.insp.mx/salud/index.html
Key words: environmental mutagens; genotoxicity test; Comet assay; Tradescantia; Mexico
Alvarez-Moya C, Santerre-Lucas A, Zúñiga-González G, Torres-Bugarín O, Padilla-Camberos E, Feria-Velasco A.
Evaluación de la genotoxicidad de hidrazida málica, N-nitroso dietilamina, y etil metano sulfonato, en núcleos de Tradescantia, por medio de la prueba del cometa.
Salud Publica Mex 2001;43:563-569.
El texto completo en inglés de este artículo también está disponible en: http://www.insp.mx/salud/index.html
Resumen
Objetivo. Evaluar la genotoxicidad de N-nitroso dietilamina (NDEA), hidrazida málica (MH) y etil metano sulfonato (EMS), en núcleos de Tradescantia (clona 4430) por medio de la prueba del cometa y de la prueba de mutación rosa, en los pelos estaminales de la misma planta. Material y métodos. Las plantas de Tradescantia (clon 4430) fueron obtenidas del Laboratorio de Citogenética y Mutagénesis del Centro de ciencias de la Atmósfera de la Universidad Nacional Autónoma de México, tratadas con NDEA a 1, 5, 10 mM, MH a 1, 5, 10 mM y EMS a 15, 30 y 45 mM, y utilizadas en la prueba de mutación rosa y en la del cometa, en núcleos celulares de los pelos estaminales. En la primera, la lectura de los pelos estaminales se realizó de acuerdo con el método de Underbrink. En otros estudios, que han aplicado la prueba del cometa en plantas, existe la necesidad de romper la pared celular y separar los núcleos por gradiente de centrifugación; en este caso, los núcleos de las células de los pelos estaminales fueron extraídos por aplastamiento sin aplicar un procedimiento especial para romper la pared, colectados por filtración en una malla de nylon y sometidos a la prueba del cometa. La prueba t de Student se usó para analizar los datos obtenidos. Resultados. Ambas pruebas presentaron una gran sensibilidad a los mutágenos estudiados y hubo una relación evidente dosis-eventos rosa / longitud de la cauda. Aunque la prueba de mutación rosa en Tradescantia fue muy sensible a MH y EMS, no se detectaron dosis bajas de NDEA; en cambio, la prueba del cometa en la misma planta permite detectar fácilmente la actividad de todos los agentes estudiados. Conclusión. La prueba del cometa en los núcleos de las células de los pelos estaminales de Tradescantia es una útil herramienta para los estudios de monitoreo. Además, es simple, sensible y más rápida que la prueba de mutación rosa en la misma planta. El texto completo en inglés de este artículo también está disponible en: http://www.insp.mx/salud/index.html
Palabras clave: mutágenos ambientales; pruebas de genotoxicidad; prueba del cometa; Tradescantia; México
Techniques allowing the detection of DNA damage are excellent tools in carcinogenesis and environmental toxicology studies.1-3 One of the methods most widely used to detect genotoxic activity of chemical or physical agents is the somatic mutation test on Tradescantia staminal hairs (clone 4430).4 This test is based on the fact that Tradescantia staminal hair cells are heterozygous for color (Aa). The dominant allele (A) accounts for the blue color and the recessive allele (a) is responsible for the pink color. Mutations in the dominant allele will result in expressing the recessive allele (pink cells).5,6 This system shows a high accuracy in laboratory experiments, and is also applicable for in situ monitoring in the environment. It requires only short training times.2 The protocol for Tradescantia includes collecting data along a 9-day period, usually days 7 to 15 after treatment.5,6 The pink mutation frequency in the stamen hairs is expressed as the number of the mutant events, either per hair (usually 103 hairs) or per hair-cell division (usually 104 hair-cell division).4
One of the most recent tests for detecting genetic damage in cells is the alkaline comet assay. It allows quantification of low levels of DNA damage in individual cells, is very simple, and only requires only a few cells.7,8 This technique detects thread rupture, alkali sensitive sites, and bonding. It has been widely used in animal cells as well.7-10 The comet assay system has shown to be more sensitive than other assays in detecting mutagenicity.11 In plant cells, the single cell gel electrophoresis assay is less frequent, however, a number of studies have been reported on the induction of mutations by chemical mutagens.12-20 Koppen and Verschaeve reported the first application of the comet assay to plant cells for genotoxicological assessment on nuclei from Vicia faba. The analysis of genomic damage induced by EMS was also studied on cultured tobacco cells.14,16 Poli et al,19 applied the comet assay to human leukocytes and, with major improvements, to plant cells (Allium cepa roots and epigean tissues of Impatiens balsamina). The first findings on human leukocytes confirmed the sensitivity of this assay, its peculiarity, and its applicability in assessing genotoxicity in environmental samples. Nuclei from Vicia faba root cells were used to value repair capacity to X-ray induced DNA damage measured by the comet assay.15 All the systems, which report the use of the comet assay on plant nuclei, refer that it is necessary to break cell walls and use a centrifugation gradient to separate the nuclei.
Tradescantia staminal hair cells and their nuclei are very large and very easy to obtain. In these cells, the comet assay can be used without immersing the tissue in liquid nitrogen or centrifuging it on a density gradient to separate the nuclei.
The present work was conducted to achieve two main objectives: to standardize the comet assay on Tradescantia nuclei and use it for monitoring ambiental genotoxics; and to compare it with the Tradescantia staminal hair system.
Material and Methods
Chemical mutagens and doses applied in this experiment were: N-nitroso diethylamine (NDEA) 1, 5, and 10 mM; maleic hydrazide (MH) 1, 5, and 10 mM; and ethyl methane sulfonate (EMS) 15, 30 and 45 mM (Sigma Chemical Co., U.S.A.).
Plants
Tradescantia, (clone 4430) (hybrid T. subacaulis X T. hirsutiflora) is highly sensitive to environmental mutagens; it was provided by Laboratorio de Citogenética y Mutagénesis del Centro de Ciencias de la Atmósfera de la Universidad Nacional Autónoma de México.
To increase the efficiency of scoring, the pink mutation rate was calculated on the basis of the number of pink mutant events, divided by the average number of hairs per flower, and expressed in terms of pink mutant events per 1 000 hairs.
Treatment and Scoring in Tradescantia
Plants were grown under controlled conditions, with a 22 °C daytime temperature and a 16-18 °C nighttime temperature. For each treatment 30 inflorescences were immersed for 3 h in 250 ml of the solution to be tested. After treatment, cuts were thoroughly washed in tap water for 30 minutes and placed in a beaker with Hoagland's nutrient solution. Untreated plants immersed in Hoagland's solution were used as negative controls. For each treatment, 5-10 flowers (i.e. about 1 500 to 3 000 stamen hairs) were scored every day, depending on how many flowers were available.
The stamen hairs were observed daily from day 7 to day 15 after the application of chemicals, period which the pink mutation is evident. The mutational events as well as the number of observed hairs and cells were scored under a dissecting microscope. The frequency of pink colored cells (events per 1 000 cells) was established according to the methodology described by Underbrink et al.5 The mean values of pink mutation are shown with 95% confidence intervals (p=0.05).
Obtaining Nuclei
Staminal hair cell nuclei were separated as follows: Twenty-four plant stamens were treated as described above (this was necessary to compare both tests) and obtained day 14 after treatment were placed in a cold mortar and 440 ml of Honda buffer (0.44 M sucrose, 2.5% Ficoll (type 400), 5% Dextran T-40, 25 mM Tris-HCl (pH 8.5), 10 mM MgCl2, 10 mM b-mercaptoethanol, and 2.5% Triton X-100) were added. According the pink mutation assay, on this period the higher mutation frequency occurs. After homogenization, the mix was filtered through a nylon fabric, (80 µm mesh). The nuclei were separated by centrifugation at 3000 rpm (4 °C) for 3 minutes. A centrifugation gradient and freezing in liquid nitrogen to break the cellular wall were not necessary to separate nuclei. This section is different from the method used by Koppen and Verschaeve12 and represents an interesting simplification of their original protocol. Nuclei were washed three times in 5 ml of physiological saline solution (0.9% NaCl), resuspended in 200 ml of saline solution and preserved at -20 °C until performing electrophoresis. The rest of the methodology was conducted as described in Singh et al.7
Alkaline Comet Assay
The basic alkaline technique of Singh et al,7 was followed. Slides were covered first with 0.5 ml of Normal Melting Point (NMP) 1% agarose, which was allowed to solidify and was then removed. Immediately thereafter, 300 µl of NMP 0.6% agarose was placed on the slide and left to solidify again. Afterwards, 100 µl of Low Melting Point (LMP) 0.5% agarose was mixed with 10 µl of the nuclear suspension and also placed on the slide. A third layer consisted of 100 µl of LMP 0.5% agarose, which was added to cover the cell-containing layer. Slides were treated during 3 h at 25 °C with several concentrations of NDEA, EMS and MH, that were kept at 4 C after treatments, to avoid the repair of the damage induced by the chemical.
For nuclear lysis and to permit DNA unfolding, slides were immersed in lysis buffer (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris-HCl, 1% sodium lauryl sarcosine, 1% Triton X-100, and 10% DMSO, pH 10) for one hour at 4 C. After lysis, slides were placed in a horizontal electrophoresis system with a high pH buffer (30 mM NaOH, 1 mM Na2EDTA, pH 13) for 45 minutes, to allow unwinding of DNA before electrophoresis. This step was optimized (the incubation time of 45 minutes was found to be optimal under this condition. Electrophoresis lasted for 15 minutes at 9 volts and 200 mA. All of the steps described above were carried out under yellow light to prevent additional DNA damage. After electrophoresis, slides were washed gently to remove alkali and immersed in neutralization buffer (0.4 M Tris-HCl, pH 7.5) for 5 minutes. Gels were stained with ethidium bromide (100 µl at 20 µg/ml) for 10 minutes and then rinsed three times with distilled water. The preparation was then covered with a glass cover slip.
Fluorescence microscopy was used to observe the slides with a light microscope equipped with a 515-560 nm excitation filter. Nuclei were photographed at 10X and 40X magnifications using a 35 mm color ASA 400 film. Migration was determined by measuring the tail length from the nucleus center. A variable number of randomly selected nuclei were measured for each experimental group.7
Statistical Analysis
The effect of the chemical dose on the length of DNA migration was analyzed using a one-tailed trend test, with a significance level set at 0.05. Differences between the control values and the values of each concentration of tested compounds were investigated using the Student's t test .12
Results
Tradescantia
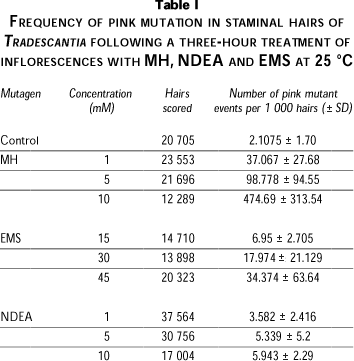
The average of pink mutation frequency from day 7 to day 15 after treatment with EMS, MH and NDEA (in duplicates) was calculated (Table I). Depending on the number of flowers available, the number of observed hairs were different in each treatment. The frequency of mutation rate of the control group was 2.1075 ± 1.70 (S.E). The three mutagens increased the mutation frequency, as compared with the control group. In both cases of EMS and MH, a lineal relationship between dose and frequency was observed. NDEA 10 mM, did no cause a frequency of mutation higher than 5 mM. MH induced the highest frequency of pink mutation at all concentrations.
Comet Assay
The methodology used by Singh et al7 showed to be effective on nuclei from Tradescantia staminal hair cells and it was optimized until it was repeatable. The condition for isolating and freezing the nuclei used here did not inflect DNA strand breaks.
Table II shows the mean tail length in nuclei of Tradescantia staminal hairs exposed to the different mutagens. Two slides from each mutagen concentration were made, however, different number of nuclei were observed. The slides exposed to MH 10 mM, NDEA 5 y 10 mM showed a low number of nuclei due to high DNA damage and the tail length was longer, thus it was not possible to make further measurements (Table II).
The mean and standard deviation of each group were calculated. A significant increase (one-tail trend, p<0.0001) in the length of DNA migration was observed in nuclei from Tradescantia staminal hairs exposed to each mutagen. The three chemical mutagens showed mutagenic activity and a relationship tail/length-concentration was observed (Table II). The higher concentrations induced high heterogeneity in DNA migration.
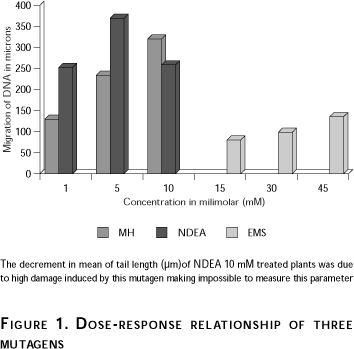
Both the pink mutation test in Tradescantia staminal hairs and the comet assay test in nuclei from Tradescantia staminal hairs detected mutagenic activity, which was different depending on the mutagen (Tables I and II). Using the pink mutation assay, NDEA showed the smallest mutagenic activity however, when the comet assay on nuclei of Tradescantia staminal hairs was used, the NDEA did show a higher mutagenic activity. A direct relationship dose-tail length of the three mutagens was observed (Figure 1). It is clear that NDEA was the strongest mutagen, followed by MH and EMS.
Discussion
The mutation assay system in Tradescantia stamen hairs has been widely used to detect agents with mutagenic activity.2,4-6,21,22 Three reference mutagens; MH, NDEA and EMS, were used in this study at well established concentrations; the results were compared and very similar to other reports.2,12-14,22,23 Using this efficient, simple and inexpensive test, experimental results could be obtained within a few days.6 The protocol with Tradescantia includes collecting data along a 9-day period, usually days 7-15 after the application of the mutagen. The comet assay system yields results within few hours and requires only extremely small cell samples.7,24,25 The single cell gel electrophoresis assay has been used in a variety of mammalian cells.7,10,26-28 Frequently, 20 nuclei are considered suitable to obtain trusty results, even though it is better to use 50-100 cells. Recently the comet assay has been used in plant cells and has been shown to be highly efficient12-19 In these reports the methodology includes breaking of the cell wall and isolating the nucleus. The comet assay using nuclei from Tradescantia staminal hairs is easier than other methods in plant tissue because it only requires to squash the staminal hairs; after that, the nuclei can be filtered (it is not necessary to isolate them using a centrifugation gradient); therefore, utilization of liquid nitrogen and a centrifugation gradient are not necessary. The next step of the methodology is the same as that reported by Singh et al.7 Moreover, the Tradescantia nuclei are bigger than other plant nuclei, so they can be easily observed. On the other hand, as the nuclei size was heterogeneous, statistical analysis showed high standard deviations, which could be diminished talking into account a higher number of cells.
The comet assay using nuclei from Tradescantia staminal hairs can be used in two ways: (a) with untreated plant nuclei to induce genetic damage in vitro and (b) with nuclei of treated plants (in vivo). We used the second option in order to apply similar experimental conditions in both assays and to be able to compare them later. Even though the in vivo comet assay takes longer than the in vitro one, it still is shorter than the classical Tradescantia pink mutation test. In this case nuclei from any day between days 10 to 14 presented a stronger mutagenic effect. When comparing the two systems, it is very important to have a clear idea of what each system is measuring; the pink hair mutation assay measures mutations, while the comet assay, at the pH used, evaluates clastogenicity. A higher migration of the tail can be compared with a higher frequency of pink events in the pink mutation assay.
The mutagenic effect of the three mutagens (EMS, MH and NDEA) as compared to their controls, was evident with both assays, but especially with the comet assay (p<0.0001). The mutagenic effect presented a close relationship with the mutagen concentration. These results are similar to other works that have been reported with the Tradescantia mutation and the comet assays, both in mammalian and plant cells, at similar mutagen concentrations. 14,16,17,22,23,29
Some difficulties arose at the highest concentrations of MH (10 mM) and NDEA (5 and 10 mM), probably due to extreme DNA damage. In these conditions, the tail length could not be measured properly. Although NDEA has been reported to be a potent mutagen, this mutagenic effect was no very clear in our experimental conditions with the Tradescantia pink mutation assay using 1,5 and 10 mM. However, when we applied the same concentrations in the comet assay on Tradescantia staminal hair nuclei, we observed greater genetic damage (the DNA dispersion was not strong, so it was difficult to measure the tail length and as a consequence a few cells were included in the statistical analysis) (Table II). On the other hand, genetic damage by MH 10 mM was more evident using the Tradescantia pink mutation assay.
Figure 1 shows a direct dose-tail length relationship of the three mutagens. As aforementioned, only a few nuclei treated with 10 mM NDEA were recorded because of the high DNA diffusion.
The comet assay on Tradescantia nuclei is as sensible as the comet assay on mammalian cells. It is also useful to monitor genotoxic agents dangerous to any organisms and not only to humans. The results of studies on plant cells can be extrapolated to animal systems and many mutagens were first detected in plants, so the importance of these assays in plants is evident.30 The present report, together with others on plant nuclei, demonstrates that it is possible to apply the comet test to any plant. This could be extrapolated to any plant cell that is suspected to be exposed to mutagens (crops exposed to pesticides, wild plants exposed to contaminants). The comet assay on Tradescantia nuclei can be a useful and economic tool when genetically dangerous genotoxic agents, are being used indoors (factories for example).
In conclusion, the comet assay in nuclei of Tradescantia is an effective, simple, and rapid procedure, which can provide valuable information in mutagenicity. It can be used to monitor environmental mutagens dangerous to humans and other organisms. This is not to say that the pink mutation assay should be discarded, but rather that the comet assay with Tradescantia staminal hair cells is useful as a complementary tool to get repeatable results in a short time.
References
1. Graf U, Würgler F, Kazt A, Frei H, Joan H, Hall C et al. Somatic mutation and recombination test in Drosophila melanogaster. Environ Mutagen 1984; 6:153-188. [ Links ]
2. Grant W, Lee H, Logan D, Salamone M. The use of Tradescantia and Vicia faba bioassays for the in situ detection of mutagens in an aquatic environment. Mutat Res 1992;2:53-64. [ Links ]
3. Ames A, Durston W, Yamasaki E, Lee F. An improved bacterial test system for the detection and classification of mutagens and carcinogens. En: Miller J, ed. Discovering Molecular Genetics. Los Angeles: Cold Spring Harbor Laboratory Press, 1996:367-376. [ Links ]
4. Ichikawa S. Tradescantia stamen hair system as an excellent botanical tester of mutagenicity: Its response to ionizing radiations and chemical mutagens and some synergistic effects found. Mutat Res 1992;270:3-22. [ Links ]
5. Underbrink A, Schairer L, Sparrow A. Tradescantia stamen hairs: A radiobiological test system applicable to chemical mutagenesis. En: Hollaender A, ed. Chemical mutagens: Principles and methods for their detection. Nueva York: Plenum Press, 1973:71-207. [ Links ]
6. Ma T, Cabrera G, Cebulska-Wasilewska A, Chen R, Loarca F, Vandenberg A et al. Tradescantia stamen hair mutation bioassay. Mutat Res 1994;310: 211-220. [ Links ]
7. Singh N, McCoy M, Tice R, Schneider L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 1988; 175:184-191. [ Links ]
8. Fairbairn D, Olive P, O´Neill K. The comet assay: A comprehensive review. Mutat Res 1995;339:37-59. [ Links ]
9. Betti C, Davini T, Giannessi L, Loprionow N, Barale R. Microgel electrophoresis assay (comet test) and SCE analysis in human lymphocytes from 100 normal subjects. Mutat Res 1994;307:323-333. [ Links ]
10. Pandrangi R, Petras M, Ralph S, Vrzoc M. Alkaline single cell gel (comet) assay and genotoxicity monitoring using blue heads and carp. Environ Mol Mutagen 1995;26:345-356. [ Links ]
11. Monteith D K, Vanstone K. Comparison of the microgel electrophoresis assay and other assays for genotoxicity in the detection of DNA damage. Mutat Res 1995;345:97-103. [ Links ]
12. Koppen G, Verschaeve L. The alkaline comet on plant cells: A new genotoxicity test for DNA breaks in Vicia faba roots cells. Mutat Res 1996; 360:193-200. [ Links ]
13. Navarrete MH, Carreara P, De Miguel M, De la Torre C. A fast comet assay variant for solid tissue cells. The assessment of DNA damage in higher plants. Mutat Res 1997;389:271-277. [ Links ]
14. Gichner P, Plewa MJ. Induction of somatic DNA damage as measured by single cell gel electrophoresis and point mutation in leaves of tobacco plants. Mutat Res 1998;401:143-152. [ Links ]
15. Koppen G, Angelis KJ. Repair of X-ray induced DNA damage measured by the comet assay in roots of Vicia faba. Environ Mol Mutagen 1998; 32:281-285. [ Links ]
16. Stavreva DA, Plewa MJ, Gichner T. Single cell gel electrophoresis analysis of genomic damage induce by ethymethanesulfonate in cultured tobacco cells. Mutat Res 1998;422:223-330. [ Links ]
17. Gichner T, Ptacek O, Stavreva AA, Plewa MJ. Comparison of DNA damage in plants as measured by single cell gel electrophoresis and somatic leaf mutations induced by monofunctional alkilanting agents. Mutat Res 1999;33:279-286. [ Links ]
18. Koppen G, Toncelli LM, Triest L, Verschaeve L. The comet assay: A tool to study alterations of DNA integrity in developing plant leaves. Mech Ageing Dev 1999;110:13-24. [ Links ]
19. Poli P, Buschini A, Restivo FM, Ficarelli A, Cassoni F, Ferrero I et al. Comet assay application in environmental monitoring: DNA damage in human lymphocytes and plant cells in comparison with bacterial and yeast tests. Mutagenesis 1999;14:547-556. [ Links ]
20. Menke M, Angelis KJ, Schubert I. Detection of specific DNA lesions by a combination of comet assay and fish in plants. Environ Mol Mutagen 2000;35:132-138. [ Links ]
21. Gichner T, Valemínsky J, Pokorny O. Somatic mutation induced by maleic hydrazide and its potassium and diethanolamine salts in Tradescantia mutation assay. Mutat Res 1982;103:289-295. [ Links ]
22. Gichner T, Veleminsky J, Rieger R. Antimutagenic effect of Diethyldiothiocarbamate toward malec hydrazide and N-Nitrosodiethylamine induced mutagenicity in Tradescantia mutagenicity assay. Biol Plantarum 1988;30:14-19. [ Links ]
23. Lijinski W. Life span and cancer: The induction time of tumors in diverse animal species treated with nitrosodiethylamine. Carcinogenesis 1993; 14:2373-2375. [ Links ]
24. Olive PL, Banáth JP, Durant RE. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the comet assay. Radiat Res 1990;122:86-94. [ Links ]
25. Mckelvey-Martin VJ, Green MHL, ScMHezer P, Pool-Zobel BL, De Méo MP, Collins A. The single cell gel electrophoresis assay (comet assay): A European review. Mutat Res 1993;288:47-63. [ Links ]
26. Sasaki YF, Saga A, Akasaka M, Nishidate E, Watanabe-Akamura M, Ohta T et al. In vivo genotoxicity of heterocyclic amines detected by a modified alkaline single cell gel electrophoresis assay in a multiple organ study in the mouse. Mutat Res 1997;395:57-73. [ Links ]
27. Speit G, Haupter S, Hartmann A. Evaluation of the genotoxic properties of paraquat in V79 Chinese hamster cells. Mutat Res 1998;412: 187-193. [ Links ]
28. Vigreux C, Poul JM, Deslandes E, Lebailly P, Godard T, Sichel F, et al. DNA damaging effects of pesticides measured by the single cell gel electrophoresis assay (comet assay) and the chromosomal aberration test in CHOK1 cells. Mutat Res 1998;419:79-90. [ Links ]
29. Gichner T, Valemínsky J, Pokorny O. Somatic mutation induced by maleic hydrazide and its potassium and diethanolamine salts in Tradescantia mutation assay. Mutat Res 1982;103:289-295. [ Links ]
30. Grant W. The present status of higher plant bioassay for the detection of environmental mutagens. Mutat Res 1994;310:175-185. [ Links ]
(1) Laboratorio de Genética, Departamento de Biología Celular y Molecular, CUCBA, Universidad de Guadalajara, Guadalajara, Jalisco, México.
(2) Laboratorio de Mutagénesis, Centro de investigación Biomédica de Occidente, IMSS, Guadalajara, Jalisco, México.
(3) Programa Internacional, Facultad de Medicina, Instituto de Ciencias Biológicas, Universidad Autónoma de Guadalajara.
(4) División de Patología y Biotecnología Ambiental, CIATEJ (SEP-CONACYT), Guadalajara, Jalisco, México.
Received on: November 9, 200 • Accepted on: July 17, 2001
Adress reprint requests to: Dr. Carlos Alvarez Moya, Agustín Melgar 1533 Chapultepec Country, Guadalajara, Jalisco, México. C.P. 44260.
E-mail: calvarez@cucba.udg.mx














