1. Introduction
The charge transport mechanism in polymeric materials has been developed increasing part of the research to know about the general theory of polymer physics [1-3]. Conducting polymer / inorganic composites were acquired unique chemical and physical properties and fascinated more attention. Due to exclusive properties conducting polymers have potential applications in materials science and electronics etc. [4-7]. So far many conducting polymers and inorganic nanoparticles have been reported in the literature review [8-10]. Among them, ZrO2 has potential applications for the fabrications of electronic devices that were described in the literature review [11]. Meanwhile, ZrO2 was an attractive material with great strength, high thermal stability, low thermal conductivity, and tremendous chemical resistance. These features make the ZrO2 very important material in the field of temperature- dependent devices applications [12].
The different metals and metal oxide have been encapsulated into conducting polymers, providing a host of composites [11-19]. Among several conducting polymers, PPy is normally known as positive conducting polymers for commercial applications due to high conductivity, simple preparation, and environmental stability but insoluble in organic solvents [20]. An active procedure to reduce this problem is to prepare the composites of conducting polymers doped with a functional dopant such as dodecylbenzene sulphonic acid (DBSA) to minimize the problems of solubility [21-22]. However, the literature review reveals that temperature- dependent DC conductivity studies of PPy doped with DBSA and also mixed with zirconium oxide are scarce. In this article, PPy-DBSA-ZrO2 composites have been synthesized by the chemical polymerization technique that provide unique properties. Temperature dependence DC Conductivity of PPy-DBSA with an increasing ratio of ZrO2 at different temperatures has been studied. The results cover density of states, hopping length; activation energy and temperature dependence DC Conductivity were calculated and discussed the acquired results. The charge transport mechanism in these synthesized materials by means of variable range hopping (VRH) model may useful for the fabrication of electronics devices.
2. Experimental details
2.1. Chemical and materials
The Pyrrole (Fluka) was purified under low pressure and stored at low temperature prior to use. Ammonium Persulphate (APS), Dodecylbenzene Sulfonic Acid (DBSA) and Zirconium oxide (ZrO2) were acquired from Sigma Aldrich also used as obtained. All materials were used as presented without any more purification.
2.2. Synthesis of polypyrrole (PPy)
Ammonium Persulphate was isolated in 100 ml of distilled water and stirred for 1h, after that the pyrrole was dissolved gradually into the mixture through stirring at room temperature. The monomer to oxidant molar ratio was retained 1:1. The overall solution was left for 24 h to attain the complete polymerization. Finally, the solution was cleaned and washed with distilled water repeatedly up to the filtrate changes into colorless shape. The greenish-black paste of PPy was achieved which was desiccated at 60°C in a vacuum oven for 24 h.
2.3. Synthesis of PPy-DBSA-ZrO2 composites
A total of 0.15 mol of DBSA was dissolved in 100 mL of distilled water, the required mol of Pyrrole was inserted gently into the mixture and the solution was kept on magnetic stirring. 30 % hydrochloric acid (HCl) was also mixed in it to maintain PH between 0 and 1. To this solution, ZrO2 powder with increasing weight percentage was added to the polymerization reaction mixture with strong magnetic stirring. After 3 h, the suitable amount of APS was dissolved in 100 mL distilled water then it was mixed dropwise under strong stirring for 1/2 h. The monomer/oxidant/dopant molar ratio was preserved 1:1:1/4. After 24 h, one-liter methanol was also introduced into the reaction mixture. The solution was left for 48 h to achieve complete polymerization. Finally, the solution was rinsed with distilled water continuously until the filtrate changes into colorless shape. The greenish-black paste ofPPy-DBSA-ZrO2 was attained which was desiccated at 60°C in a vacuum oven for 24 h.
3. Measurements
Raman spectra for 633 nm exciting radiation were detailed on a Renishaw RM 1000 laser Raman (He-Ne laser) obtaining Olympus metallurgical microscope and a CCD detector. The laser power at the sample was retained below ~ 0.74 mW to protect the thermal degradation. The laser was concentrated employing a 50 x objective lens and spatial resolution was concerning 1 μm. Scanning electron microscopy (SEM) was carried out on an EVO50 ZEISS instrument. DC conductivity of all samples was performed with Keithley 2400 electrometers and a current source electrometer by using two probe method.
4. Results and discussion
4.1. Raman spectroscopy
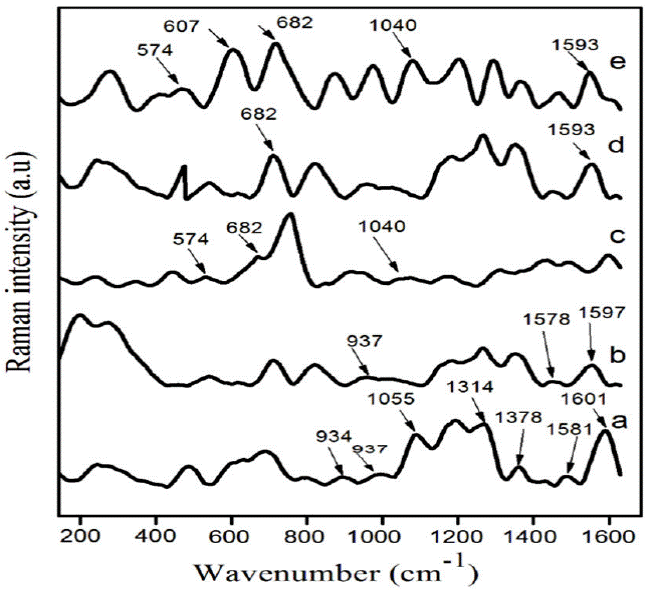
Raman spectroscopy has been used to quantify the detailed analysis of induced structural changes by adding ZrO2 in PPy-DBSA. Figure 1a) depicts the Raman spectra of polypyrrole (PPy). The peaks observed at 1597 and 1581 cm-1 represent C = C symmetry stretching [23-24]. The two peaks located at 1314 and 1378 cm-1 assigned to be the inter ring (C-C) stretching [22-25]. The peak at 1055 cm-1 is attributed to ring deformation and two another peaks were located at 937 and 934 cm-1 are seen due to ring deformation related to bipolarons and polarons [26]. Figure 1b) shows the Raman spectra of PPy doped with DBSA. The peak at 1578 cm-1 is observed due to benzenoid C-C ring stretching vibrations and another peak located at 1601 cm-1 showing the quinoid C=C stretching mode of the polymer chain. Figure 1 c)-e) shows that the peaks of PPy-DBSA-ZrO2 composites were found at 1593 cm-1 (N-C stretching band), 1040 cm-1 (N-H in-plane deformation) and 682 cm-1 (C-C out of plane ring deformation). Moreover, two peaks observed at 607 and 574 cm-1 are related to benzene ring deformation [27] and cross-linking between the PPy chains [28] respectively. The peak at 607 cm-1 has been attributed to the deformation of the benzene ring in the PPy backbone. Figure 1e) illustrates that the intensity of the peak at 607 cm-1 increases as compared to the peak observed at 574 cm-1 provides information regarding the interactions between polymer components. The literature showing that it was due to the fact that, by inserting ZrO2 displayed a good attraction between the PPy chains [29]. The relative intensity of the peak at 607 cm-1 indicates that the ZrO2 mixed with PPy-DBSA confirms the inter chain interaction. This effect was betterly observed in composite an with 8 % weight ratio of ZrO2 into PPy-DBSA. From this study, it may be concluded that the observed changes in the recorded spectra of new peaks in PPy-DBSA-ZrO2 composites predict the formation of chemical bonding present between PPy-DBSA chains and ZrO2.
4.2. Scanning electron microscopy (SEM) analysis
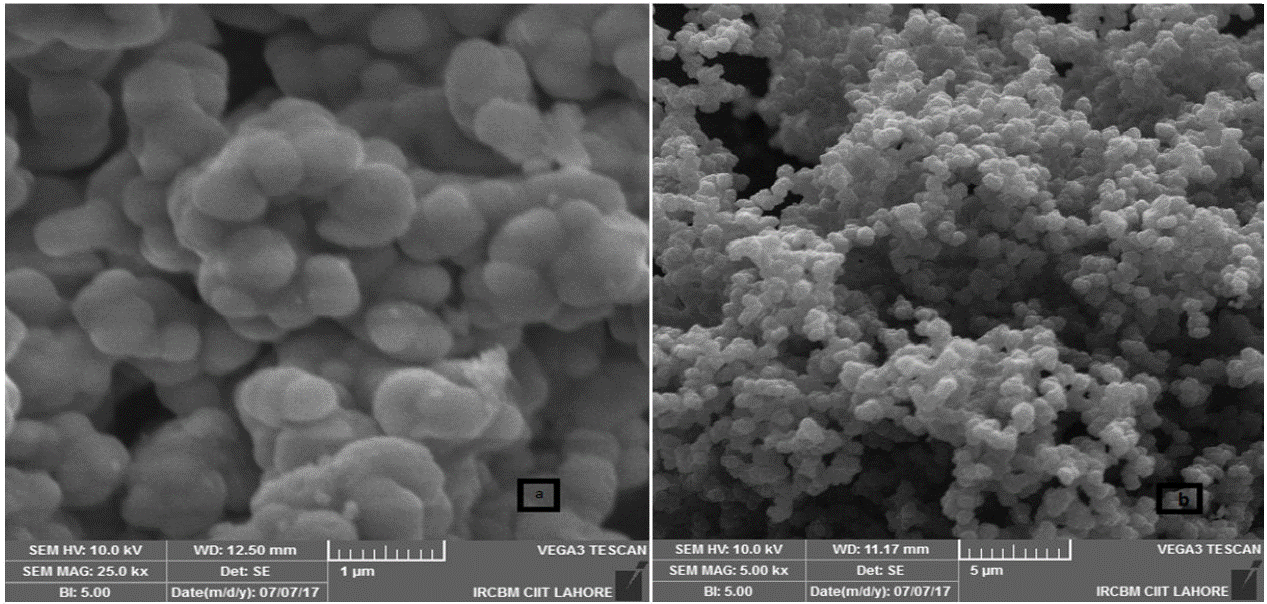
The SEM micrograph of pure ZrO2 nanoparticles and PPy-DBSA-ZrO2 composite can be acquired and are shown in Fig. 2 a)-b). It is clearly observed from the SEM image of polypyrrole (PPy) that it has clusters of spherical shaped particles. At low magnification, SEM of PPy- dodecylbenzene sulphonic acid (DBSA) displays egg shell like surface morphology that may have resulted from phase segregation. The SEM morphology of PPy-DBSA also shows the aggregations of particles which may be due to the increased in inter chain interaction and results to increases the electrical conductivity [30]. At high magnification, SEM image is indicative of hemi spherical nature of polymer as clusters in PPy-DBSA-ZrO2 composite as well as the platelet structure of ZrO2. The ZrO2 nanoparticles are implanted into PPy-DBSA chain because due to the strong particle interaction [31]. From this, it may be concluded that PPy-DBSA-ZrO2 is seeing more growth in particle size and a duel structure of platelet and eggshell.
4.3. Temperature dependent DC conductivity of PPy-DBSA-ZrO2 composites
4.3.1. Amount of ZrO2 added
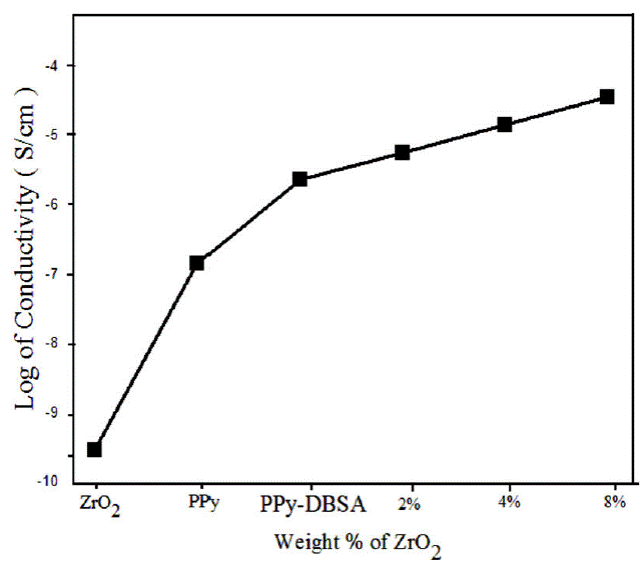
A chain of composites with different amount of ZrO2 (2%, 4% and 8%) were synthesized by retaining the concentrations of pyrrole constant. The different amount of ZrO2 generates composites of changed conductivity. The conductivities of the composites were found to be decreases with increasing amount of ZrO2 (Fig. 3). The decrease in conductivity with an accumulation of ZrO2 may be expected to reducing the conductive pathways because of the insulated nature of ZrO2. The accumulation of ZrO2 reduces degree of conjugated π-bonds in PPy in addition to terminates the ordered structure of polymer chains which effects to decrease in conductivity of the composite. Hence, the existence of ZrO2 plays a very significant part in the conductivity of PPy-DBSA-ZrO2.
4.3.2. Temperature dependant DC conductivity
The relation concerning DC conductivity and temperature in the polymer samples can provide imperative information about the nature of phenomena correlated to the charge transport into a polymer system.
Here the exponent n in Eq. (1) [32-33] is dimensionality of system, its value can be n = 1, 2 or 3 for 1-D, 2-D or 3-D VRH charge transport system. The effective dimensionality n of charge transport based on inter chain coupling. Temperature-dependent Log of conductivity was observed to have a linear relationship to T -1/4 in case of PPy [34], PPy-DBSA and PPy-DBSA-ZrO2 composites. This implies that the charge transport method of these samples follows the 3D variable range hopping (VRH) model. The activation energy is defined as [35].
The differential of the graph of Logσ vs 1/kT offers activation energy at different temperatures. The Arrhenius exponential law equation is [36].
By adding Eqs. (2) and (3) we get the following Eq. (4).
Where
Table I Mott parameters of PPy-DBSA doped with ZrO2 (2, 4 and 8 %) at 303 K.
| Samples | T0 (K) | Density of states N(EF) | Hopping length (cm) | Hopping activation energy | σ dc (S/cm) |
| (eV-1cm-3) | (R) | (eV) (W) | |||
| PPy | 8.14 x 109 | 9.55 x 1023 | 8.17 x 10-9 | 0.458 | 2.3 x 10-4 |
| PPy-DBSA | 8.05 x 109 | 9.66 x 1023 | 8.12 x 10-9 | 0.456 | 2.64 x 10-4 |
| PPy-DBSA-ZrO2 (2%) | 5.02 x 109 | 1.54 x 1024 | 7.25 x 10-9 | 0.407 | 2.77 x 10-4 |
| PPy-DBSA-ZrO2 (4%) | 5.38 x 109 | 1.44 x 1024 | 6.15 x 10-9 | 0.414 | 3.14 x 10-4 |
| PPy-DBSA-ZrO2 (8%) | 3.52 x 10-9 | 2.21 x 1024 | 6.63 x 10-9 | 0.372 | 3.38 x 10-4 |
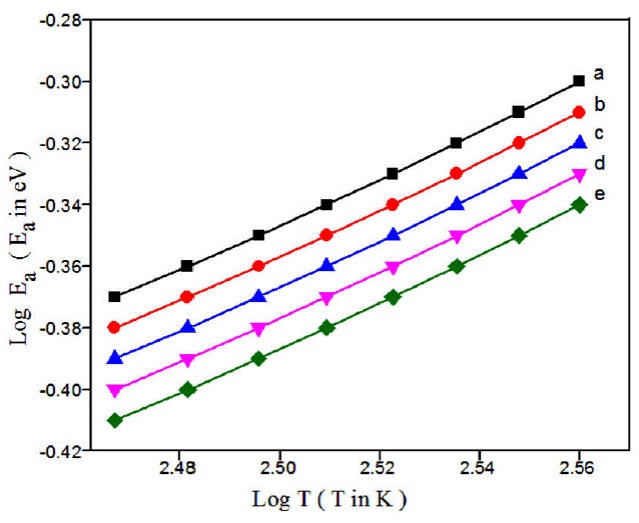
This activation energy may also be misused to see the charge transport hopping mechanism. Temperature dependent exponent n of either equal to 0.25 [37-39] or 0.5 [40-42] for various morphologies of conducting polymers. For strong inter chain coupling, exponent γ is 0.25 and effective dimensionality n = 3 for temperature dependent DC conductivity data will follows a 3D-VRH model. For weak inter chain coupling, VRH exponent is 0.5 (n = 1) which could describe in terms of a granular metal model or quasi 1D [43] or Afros' Shklovskii [44] VRH models. For PPy-DBSA-ZrO2 by plotting Log(E a) vs LogT (Fig. 4) a straight line of slope {-(γ - 1)} equal to 0.756 is obtained which resembles the hopping exponent γ ~ 0.25 and n ~ 3. This confirms that 3D-VRH dominates the mechanism of charge transport in PPy-DBSA-ZrO2 composite same as 3D-VRH model apply in PPy [34]. The activation energy attained from Arrhenius plot increases with increase in temperature. Therefore, Log (𝜎) having linear relationship with T -1/4 as shown in Fig. 5. From these results, we observed that strong inter chain interaction present in PPy-DBSA as well as in PPy-DBSA-ZrO2 samples. But decrease in DC conductivity of clay sample is due to insulating behavior of ZrO2 nanoparticles [45].
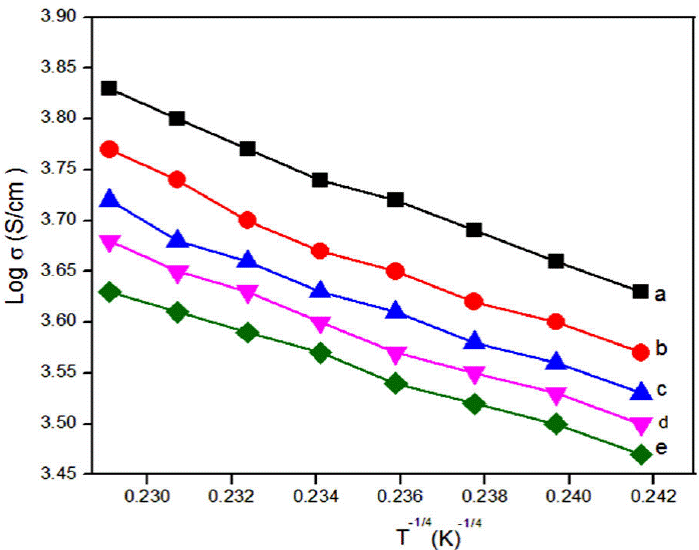
Figure 5 DC conductivity (σDC ) as a function of T -1/4 ina logarithmic scale for (a) PPy (y = -15.60𝔵 + 7.39, R2 = 0.996), (b) PPy-DBSA (y = -15.56 𝔵 + 7.32, R2 = 0.983), (c) PPy-DBSA-2% ZrO2 (y = -14.46 𝔵 + 7.06, R2 = 0.989), (d) PPy-DBSA-4%ZrO2 (y = -14.06 𝔵 + 6.89, R2 = 0.992), (e) PPy-DBSA-8% ZrO2 (y = -12.64 𝔵 + 6.52, R2 = 0.997).
5. Conclusions
The composites of doped Polypyrrole (PPy) with Dodecyl-benzenesulphonic acid (DBSA) and also mixed with ZrO2 nanoparticles were synthesized by chemical polymerization technique. The structural properties of synthesized samples were studied by Raman spectroscopy. SEM micrographs show that the composites are in the form of extended chains and increase in the particles size as compared with PPy and ZrO2 is also observed. The addition of ZrO2 disturbed the de-localization of charge carriers and decrease the inter chain interaction due to which DC conductivity of PPy-DBSA-ZrO2 (σ dc = 3.38 X 10-4 S/cm at room temperature) is higher as compared to PPy-DBSA (σdc = 2.64 X 10-4 S/cm at room temperature). Temperature dependant DC conductivity displayed three dimensional variable ranges hopping (3D-VRH) model. Density of states, hopping length, and activation energy were calculated and seen to be changed by growth of ZrO2 into PPy-DBSA.











 nueva página del texto (beta)
nueva página del texto (beta)