1. Introduction
The compounds with ternary chalcogenide structures of the chalcopyrite family I-III-VI2 (where I = Cu or Ag; III = Al, Ga or In; and VI = S, Se or Te), form a wide group of semiconductor materials with diverse optical and electrical properties [1]. They crystallize with tetragonal symmetry in the space group I
In particular, the ternary chalcopyrite semiconductor CuInSe2 has emerged as a leading material for the preparation of photovoltaic devices due to their potential applications in solar cell technology [4]. In studying the defect physics of CuInSe2, Zhang et. al. [5] have suggested that the presence of the donor-acceptor defect pair (2V1-Cu + In2+Cu) could be used to explain the existence, according Cu n-3 In n+1 Se 2n , of CuIn5Se8 (n = 4), CuIn3Se5 (n = 5), Cu3In7Se12 (n = 6), Cu2In4Se7 (n = 7), Cu5In9Se16 (n = 8), Cu3In5Se9 (n = 9) as due to the repeat of a single unit of this defect pair for each n = 4, 5, 6, 7, 8 and 9 units, respectively, of CuInSe2. These new materials, which have a deficiency of cations over the anions, belongs to the defect semiconductor families [3], and are known as ordered vacancy compounds (OVC’s) [6].
Similarly we can define Ag-content derivatives, from the chalcopyrite AgInTe2, and the existence of several of these compositions has been shown from the phase diagrams analysis of the AgTe-In2Te3 pseudo-binary system [7,8], such as AgIn5Te8 (n = 4), AgIn3Te5 (n = 5) and Ag3In5Te9 (n = 9), which can be written as AgIn5□2Te8, AgIn3□Te5 and Ag3In5□Te9, where □ represents the cation vacancy. The semiconducting behavior of these OVC’s has been explained, based on the four electrons-per-site rule, by assigning to this vacancy a zero valent atom [5].
While Cu-content OVC’s are of great interest for thermoelectric applications [9], the Ag-content compounds are potential candidates for several applications as photo-absorbers in solar cells, opto-electronics devices and photo-electrochemical cells [10], Concerning the crystal structures in the Ag-In-Te system, the ternary compositions AgInTe2 [11], AgIn5□2Te8 [12] and AgIn3□Te5 [13] crystalize in tetragonal structures with space groups I
Therefore, in this work, we report a detailed structural analysis of the new ternary compound Ag3In5□Te9, which crystallize with a P-chalcopyrite structure [15]. From this study, unit cell parameters, atomic coordinates, isotropic temperature factors and other relevant geometric data could be determined using X-ray powder diffraction data.
2. Experimental
The sample was synthesized by direct fusion of stoichiometric quantities of Ag, In and Te (99.99%) in a sealed, evacuated quartz ampoule and subsequent quenching in water. The mixture was slowly heated up to 523 K at a rate of 4 K/min. The ampoule was kept at this temperature for a period of 6 hours in order to allow the exothermic reaction between In and Te. Then, the temperature was gradually raised at the same rate up to 773 K. It was kept at this condition for 24 hours. Subsequently, it was heated up to 1323 K, which is higher than the melting points of the constituent elements, and kept at this temperature for 7 days. The charge in the ampoule was allowed to react in this molten state for 60 h with intermittent shaking in order to improve the homogeneity. Finally, the ampoule with the charge in the molten state was quenched in water. The chemical composition of Ag3In5Te9 crystals was determined by energy dispersive spectroscopic (EDS) analysis using a JSM-6400 electron microscope. The atomic compositions of the studied sample are: Ag (17.0%), In (29.9%) and Te (53.1%), from three different regions of the ingot. These percentages are in good quality agreement with the ideal composition 3: 5: 9. For the X-ray analysis, a small quantity of the sample, cut from the ingot, was ground mechanically in an agate mortar and pestle. The resulting fine powder was mounted on a flat holder covered with a thin layer of petroleum jelly. The X-ray powder diffraction data was collected at 293(1) K, in θ=2θ reflection mode with Bragg-Brentano geometry, using a Panalytical X’pert diffractometer equipped with an X-ray tube (CuKα radiation: λ= 1.5418 Å; 40kV, 30 mA). The specimen was scanned from 10-100 ° 2θ, with a step size of 0.02 ° and counting time of 20 s. Silicon (SRM-640) was used as an external standard. The Panaytical X’pert Pro analytical software was used to establish the positions of the peaks.
3. Results and discussion
The X-ray diffractogram of Ag3In5□Te9 shows a single phase. The peak positions were extracted by means of single-peak profile-fittings carried out with the WinPLOTR software [16].The first 20 peak positions measured from the pattern were input to the auto-indexing program DICVOL04 [17].
A unique solution was obtained in a tetragonal system with unit cell parameters a = 6.186(1) Å, c = 12.365(2) Å. The lack of systematic absence condition h + k + l in the general reflections of the type hkl indicating a P -type cell and rule out the I-type cell of the chalcopyrite structure. In addition, the conditions hhl: l = 2n and 00l: l = 2n suggests the extension symbol P
For the Rietveld refinement [19], the whole diffraction data was used. This was carried out using the Fullprof program [20] available in the software package WinPLOTR [15]. The atomic coordinates of the Cu3In5□2Te9 compound [17], were used as starting parameters and the unit cell parameters were those obtained from indexing. The angular dependence of the peak full width at half maximum (FWHM) was described by the usual constrain imposed by the Caglioti’s formula [21]. Peak shapes were described by the parameterized Thompson-Cox-Hastings pseudo-Voigt profile function [22]. The background was described by the automatic interpolation of 67 points throughout the whole pattern. With the diffraction data available it was only possible to described the thermal motion of the atoms by one overall isotropic temperature factor.
The final figures of merit for 23 instrumental and structural variables were: R p = 5.4%, R wp = 5.8%, R exp =5.1%, for 4501 step intensities and 152 independent reflections. The final Rietveld plot is shown in Fig. 1. Unit cell parameters, atomic coordinates, isotropic temperature factor, bond distances and angles are listed in Table I.
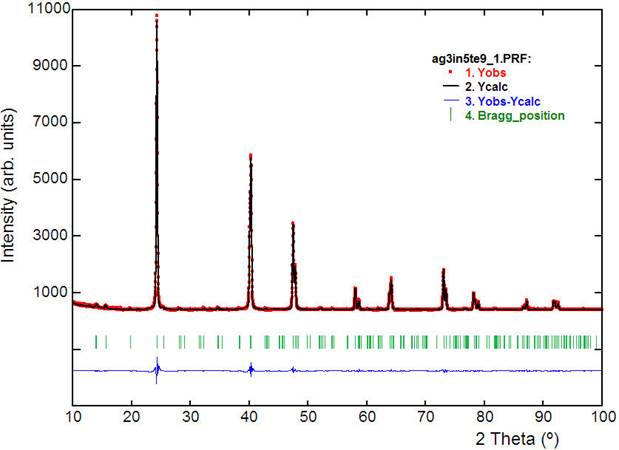
Figure 1 Final Rietveld refinement plot showing the observed, calculated and difference pattern for Ag3In5□Te9. The Bragg re-flections are indicated by vertical bars.
Table I Rietveld refinement details for Ag3In5□Te9.
| Molecular formula | Ag3In5□Te9 | wavelength (CuKα) | 1.5418 Å |
| Molecular weight (g/mol) | 1973.44 | data range 2θ( ° ) | 10-100 |
| a (Å) | 6.3453(2) | step size 2θ ( ° ) | 0.02 |
| c (Å) | 12.5754(7) | counting time (s) | 20 |
| c/a | 1.98 | step intensities | 4501 |
| V (Å3) | 506.32(4) | independent reflections | 152 |
| Z | 0.889 (8/9) | Rp (%) | 5.4 |
| Crystal system | tetragonal | Rwp (%) | 5.8 |
| Space group | P |
Rexp (%) | 5.1 |
| dcalc (g/cm -3 ) | 6.47 | RB (%) | 4.7 |
| Temperature (K) | 298(1) | S | 1.1 |
The structure of the ternary phase Ag3In5□Te9 is a P-chalcopyrite-like compound [18], and can be described as a derivative of the sphalerite structure. In this structure the anions (Te) form a close-packed arrangement with the tetrahedral sites occupied by the cations (Ag, In) and vacancies (□) in a partially ordered fashion. This array is expected for adamantane compounds [3] and can be observed in the unit cell diagram of this ternary chalcogenide compound (Fig. 2).
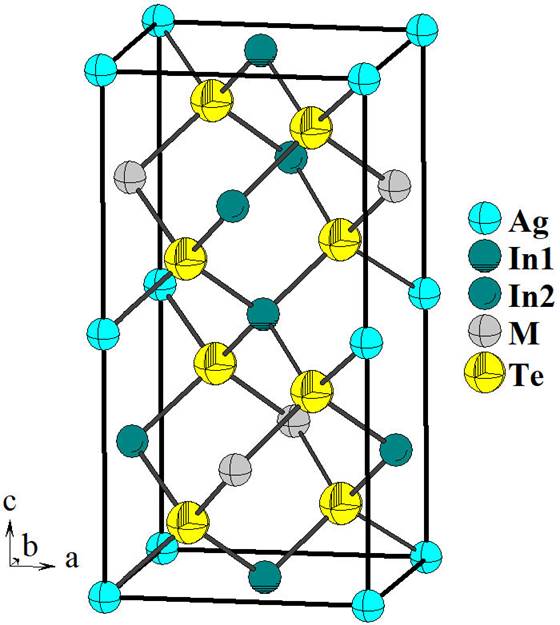
Figure 2. Unit cell diagram for the new P-chalcopyrite compound Ag3In5□Te9 (P
In the P-chalcopyrite structure, the introduction of additional cations in the crystal lattice of the chalcopyrite parent produces a degradation of symmetry from the space group I
The tetrahedra containing the Ag atoms [mean Te. . . Te distance 4.584(7) Å] are lightly smaller than those containing the In2 atoms [mean Te . . . Te distance 4.532(7) Å], M [mean Te . . . Te distance 4.498(7) Å] and In1 atoms [mean Te . . . Te distance 4.288(7) Å], respectively.
Table II Atomic coordinates, occupancy factors, isotropic temperature factors, bond lengths (Å) and angles ( ° ) for Ag3In5□Te9, derived from the Rietveld refinement.
| Atom | Ox. | Wyck. | x | y | z | foc | B (Å2) |
| Ag1 | +1 | 2e | 0 | 0 | 0 | 1 | 0.4(2) |
| In1 | +3 | 2f | 1/2 | 1/2 | 0 | 1 | 0.4(2) |
| In2 | +3 | 2d | 0 | 1/2 | 1/4 | 1 | 0.4(2) |
| Ag2 | 2b | 0.333 | 0.4(2) | ||||
| In3 | 2b | 1/2 | 0 | 1/4 | 0.222 | 0.4(2) | |
| □ | - | 2b | 0.445 | - | |||
| Te | -2 | 8n | 0.2637(7) | 0.2608(7) | 0.1217(5) | 1 | 0.4(2) |
| Ag1-Te | 2.897(5) | In1-Te | 2.626(5) | In2-Te | 2.776(5) | ||
| Te i -Ag1-Te | 107.3(1) | Te ii -Ag1-Te iv | 113.9(1) | Te iii -Ag1-Te iv | 107.3(1) | ||
| Te ii -Ag1-Te iii | 107.3(1) | Te iii -Ag1-Te | 113.9(1) | Te-Ag1-Te iv | 107.3(1) | ||
| Te viii -In1-Te | 108.7(1) | Te viii -In1-Te x | 109.9(2) | Te-In1-Te x | 108.7(1) | ||
| Te viii -In1-Te ix | 108.7(1) | Te-In1-Te ix | 109.9(2) | Te ix -In1-Te x | 108.7(1) | ||
| Te-In2-Te v | x2 105.9(2) | Te-In2-Te vi | x2 113.7(2) | Te-In2-Te vii | x2 108.9(1) | ||
Symmetry codes: (i) 1 - x, -y, z; (ii) -y, x, -z; (iii) -x, -y, z; (iv) y, -x, -z; (v) -x, y, 0:5 - z; (vi) x, 1 - y, 0:5 - z; (vii) -x, 1 - y, z; (viii) y, 1 - x, -z; (ix) 1 - x, 1 - y, z; (x) 1 - y, x, -z.
The Ag-Te [2.897(5) Å] and In-Te [2.701(5) Å] bond distances compare quite well with those observed in other adamantane structure compounds such as AgInTe2 [11], AgIn5□2Te8 [12], AgIn3□Te5 [13], CuInTe2 [25], CuTa2InTe4 [26], Ag2FeGaTe4 [27], CuCo2InTe4 and CuNi2InTe4 [28], Ag2SnTe3 [29] and Cu3In7□2Te12 [30]. Table III shown the unit cell and bond lengths comparison between the four compounds of the Ag-In-Te system reported so far.
Table III Comparative table of space group, unit cell parameters and bond distances for the ternary compounds of the Ag-In-Te system reported so far ([*] = this work).
| Space group | Unit cell (Å) | c/a | V (Å3) | Ag-Te (Å) | In-Te (Å) | Ref. | |
| AgInTe2 | I |
6.4431(4) | 1.96 | 524.57(6) | 2.811(2) | 2.734(2) | [11] |
| 12.6362(9) | |||||||
| AgIn5□2Te8 | P |
6.1952(2) | 2.00 | 476.7(2) | 2.890(6) | 2.764(6) | [12] |
| 12.419(4) | |||||||
| AgIn3□Te5 | P |
6.2443(8) | 2.00 | 487.62(9) | 2.7243(4) | 2.7052(4) | [13] |
| 12.5058(4) | |||||||
| Ag3In5□Te9 | P |
6.3453(2) | 1.98 | 506.32(4) | 2.897(5) | 2.701(5) | [*] |
| 12.5754(7) |
4. Conclusions
The new ordered vacancy compound Ag3In5□Te9 was obtained as a single phase and its structure was refined by the Rietveld method using X-ray powder diffraction data. This compound is isostructural with Cu3In5□Te9, and crystallizes in the tetragonal space group P











 nueva página del texto (beta)
nueva página del texto (beta)


