1. Introduction
The materials more frequently studied in optoelectronic and magnetic devices [1] are the known as semimagnetic semiconductors, obtained from the tetrahedrally coordinated derivatives of the II-VI binaries [2]. One of these families are the quaternary semiconductors with formula I2-II-IV-VI4, which satisfy the rules of adamantane compound formation [2] and belong to the two possible normal-valence families of fourth derivatives of the II-VI binary semiconductors with three types of cations, the other family being I-II2-III-VI4 [3]. Due to the great variety of possible compositions (I= Cu, Ag, II= Zn, Cd, Mn, Fe, III= Al, Ga, In, IV= Si, Ge, Sn, VI= S, Se, Te), these quaternary diamond-like materials have drawn wide interest for their potential application as solar-cell absorbers [4-6], photocatalysts [7], thermoelectrics [8], spintronics [9], non-linear optics [10] and magneto-optic properties when alloyed with Mn [11]. The quaternary compounds Cu2ZnSnS4, (CZTS) and Cu2ZnSnSe4 (CZTSe) are thin-film solar-cell absorbers, which have shown conversion efficiencies as high as 10% [5,6]. Furthermore, due to the promising applications in low-cost and high performance photovoltaic and thermoelectric devices, there has been an increase of syntheses of colloidal I2-II-IV-VI4 nanocrystals during the past ten years [12].
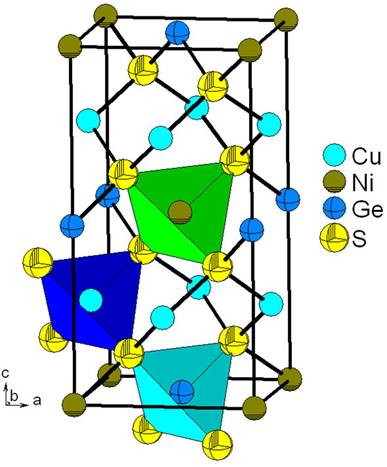
Structural studies carried out on some members of the I2-II-IV-VI4 family indicate that they crystallize in sphalerite derivative structures or wurtzite derivative structures. In sphalerite derivatives with tetragonal symmetry; in a Cu2FeSnS4-type structure (stannite, space group I42m) or in a Cu2ZnSnS4-type structure (kesterite, space group I4) [13]. In würtzite derivatives with orthorhombic symmetry in a Cu2CdGeS4-type structure (würtzite-stannite, space group Pmn21) [14] or with monoclinic symmetry in a Na2ZnSiO4-type structure (würtzite-kesterite, space group Pc) [15]. All this structure names are generally accepted types [16]. These crystallographic forms are very close with the only difference in the distribution of the cations in the tetrahedral sites (Fig. 1).
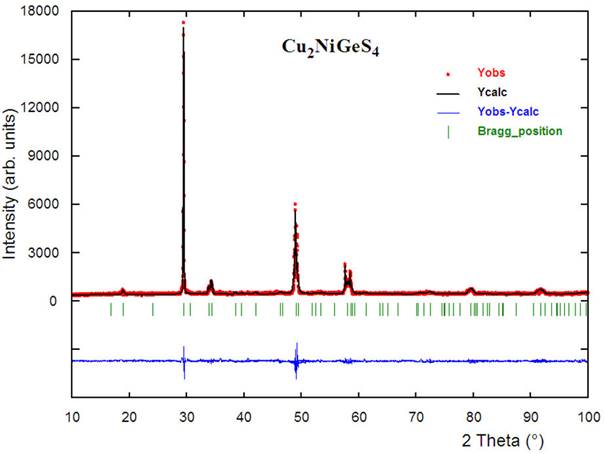
Figure 1 Unit cell diagram of the stannite, kesterite, würtzitestannite and würtzite-kesterite structures showing the cation and anion distribution in the I2-II-IV-VI4 family.
Among this family of compounds, Cu2iGeS4 is of considerable interest and their preparation and study was reported some time ago [17-19]. However, very little work on its physical properties appears in the literature. Only one study on their magnetic properties indicated that this material is antiferromagnetic with a Néel temperature of 36 K and a magnetic moment of 2.7 MB [17]. From the crystallographic point of view, in earlier studies using Güinier data, was designated as “orthorhombic deformed” cell for this phase with unit cell parameters a = 5.34 Å, b = 5.27 Å, c = 10.47 Å [18], and few years later the same author reported a “sphalerite deformed” cell with unit cell parameters a = 5.332 Å, b = 5.263 Å, c = 5.227 Å [19] however, these works were not conclusive because of the poor quality of the diffraction pattern (PDF 26-549) [20].
In recent years, it has been of interest to carry out a systematic study of the crystal structure of quaternary diamond-like families [21-32]. Hence, here we report the structural characterization of the quaternary compound Cu2NiGeS4 using the Rietveld method from X-ray powder diffraction data, with the purpose of establishing unequivocally its crystal structure and report better X-ray powder diffraction data.
2. Experimental
2.1. Synthesis
Sample of Cu2NiGeS4 was prepared by melting of pure elements (Cu, Ni, Ge, and S) in evacuated closed silica ampoules at a maximum temperature of 1100°C. The resultant polycrystalline product was ground and introduced into a new quartz ampoule, 18 cm long with a suitable amount of I2 used as transporting agent. The crystals were grown by placing the ampoule in a two-zone furnace, and keeping the source zone temperature at 1000-950°C and the deposition zone temperature 900-875°C for 15 days. Finally, the sample was cooled to room temperature at a rate of 10°/h.
The stoichiometric relation of the sample was investigated by SEM technique, using a Hitachi S2500 microscope equipped with a Kedex EDX accessory. Three different regions of the ingot were scanned, and the average atomic percentages are: Cu (25.3%), Ni (11.2), Ge (11.3%) and S (52.2%). The error in standardless analysis was around 5%. These values are in good agreement with the ideal composition 2:1:1:4.
2.2. X-ray powder diffraction
For the X-ray analysis, a small quantity of the sample, cut from the ingot, was ground manually in an agate mortar and pestle. The resulting fine powder, sieved to 106 μ, was mounted on a flat zero-background holder covered with a thin layer of petroleum jelly. The X-ray powder diffraction data was collected at 293(1) K, in Θ/2Θ reflection mode using a Siemens D5005 diffractometer equipped with an X-ray tube (CuKα radiation: λ = 1.54059 Å; 40 kV, 30 mA) and a Ge<111> primary monochromator. A fixed aperture and divergence slit of 1 mm, a 1 mm monochromator slit, and a 0.1 mm detector slit were used. The specimen was scanned from 10-100° 2Θ, with a step size of 0.02° and counting time of 40 s. Quartz was used as an external standard. The analytical software was used to establish the positions of the peaks.
3. Results and Discussion
The X-ray powder diffractogram of Cu2NiGeS4 shows a single phase. The 16 peak positions were indexed using the program Dicvol04 [33], which gave a unique solution in a tetragonal cell. The systematic absences study (hkl: h+k+l = 2n) indicated an I-type cell. The orthorhombic and monoclinic primitive cells were ruled out, discarding the würtzite-like structures. A revision of the diffraction lines taking into account the sample composition, unit cell parameters as well as the body center cell suggested that this material can crystallize with stannite-type structure in the tetragonal space group I42m (N° 121) or with a kesterite-type structure in the tetragonal space group I4 (N° 82) [13]. In the stannite structure Cu2FeSnS4 (I42m), Cu atoms are ordered to the Wyckoff 4d site, Fe atoms are ordered to Wyckoff 2a, Sn is ordered to 2b. For comparison, the I4 symmetry, in kesterite structure Cu2ZnSnS4, has Cu atoms ordered to two sites: 2a and 2c, Zn ordered to 2d, Sn ordered to 2b. Stannite exhibits one layer of Cu atoms only, with the other layer consisting of ordered Fe and Sn atoms, while kesterite exhibits one layer of ordered Cu and Sn atoms and one layer of ordered Zn and Cu atoms. It should be mentioned that a Rietveld refinement [34] was performed in the I4 (N° 82) space group but did not produce a chemically sound structure, discarding a kesterite structure.
The complete powder diffraction dataset was reviewed in the tetragonal space group I42m by using the program NBS*AIDS83 [35]. From this analysis, the refined unit cell parameters obtained were: a = 5.3308(4) Å and c = 10.5849(9) Å, with figures of merit M16 = 70.7 [36] and F16 = 320.6 (0.0101, 752 [37]. The resulting X-ray powder diffraction data for Cu2NiGeS4, together with the observed and calculated 2Θ, the d-spacings as well as the relative intensities of the reflections, are given in Table I.
Table I X-ray powder diffraction data of the quaternary Cu2NiGeS4.
| 2Θobs(°) | dobs(Å) | (I/I0)obs | h | k | l | 2Θcal(°) | dcal(Å) | Δ2Θ(°) |
| 18.631 | 4.7588 | 2.1 | 1 | 0 | 1 | 18.621 | 4.7611 | -0.009 |
| 29.066 | 3.0697 | 100.0 | 1 | 1 | 2 | 29.060 | 3.0703 | -0.006 |
| 33.581 | 2.6665 | 2.0 | 2 | 0 | 0 | 33.596 | 2.6654 | 0.014 |
| 33.841 | 2.6466 | 5.1 | 0 | 0 | 4 | 33.847 | 2.6462 | 0.005 |
| 41.659 | 2.1663 | 5.5 | 1 | 1 | 4 | 41.668 | 2.1658 | 0.009 |
| 45.903 | 1.9754 | 1.2 | 2 | 1 | 3 | 45.903 | 1.9754 | 0.000 |
| 48.241 | 1.8850 | 32.8 | 2 | 2 | 0 | 48.247 | 1.8847 | 0.006 |
| 48.423 | 1.8783 | 15.9 | 2 | 0 | 4 | 48.433 | 1.8779 | 0.010 |
| 57.656 | 1.5975 | 12.1 | 1 | 1 | 6 | 57.644 | 1.5978 | -0.012 |
| 58.244 | 1.5828 | 9.6 | 2 | 1 | 5 | 58.237 | 1.5830 | -0.007 |
| 70.604 | 1.3330 | 1.1 | 4 | 0 | 0 | 70.619 | 1.3327 | 0.015 |
| 71.202 | 1.3232 | 1.2 | 0 | 0 | 8 | 71.209 | 1.3231 | 0.007 |
| 78.792 | 1.2137 | 2.0 | 4 | 1 | 3 | 78.769 | 1.2140 | -0.023 |
| 80.503 | 1.1921 | 1.6 | 4 | 2 | 0 | 80.514 | 1.1920 | 0.011 |
| 90.675 | 1.0830 | 1.7 | 2 | 2 | 8 | 90.685 | 1.0829 | 0.010 |
| 92.853 | 1.0632 | 1.5 | 4 | 0 | 6 | 82.835 | 1.0634 | -0.018 |
The Rietveld refinement [34] performed in the I42m space group was carried out using the Fullprof program [38]. The atomic coordinates of Cu2FeSnS4 [13] were used as starting model for the quaternary Cu2NiGeS4. The angular dependence of the peak full width at half maximum (FWHM) was described by the Caglioti’s formula (FWHM2 = U tan2 Θ + V tanΘ + W where U, V and W) are fitting parameters [39]. Peak shapes were described by the parameterized Thompson-Cox-Hastings pseudo-Voigt profile function [40]. The background variation was described by a polynomial with six coefficients. The thermal motion of the atoms was described by one overall isotropic temperature factor. The results of the Rietveld refinement are summarized in Table II. Figure 1 shows the observed calculated and difference profile for the final cycle of Rietveld refinement. Atomic coordinates, isotropic temperature factor, bond distances and angles are shown in Table III.
Table II Rietveld refinement results for Cu2NiGeS4.
| Molecular formula | Cu2NiGeS4 | Wavelength (CuKα) (Å) | 1.54059 |
| Molecular weigth (g/mol) | 386.66 | Range 2Θ (°) | 10-100 |
| a (Å) | 5.3384(1) | Step size (°) | 0.2 |
| c (Å) | 10.5732(3) | Counting Time (s) | 40 |
| V (Å3) | 301.32(3) | N° intensities | 4501 |
| η = c/2a | 0.99 | Independent reflections | 78 |
| System | tetragonal | Rexp (%) | 8.6 |
| Space group | I42m (N° 121) | RB (%) | 9.5 |
| Z | 2 | Rp (%) | 9.2 |
| Dcalc (g/cm-3) | 4.261 | Rwp (%) | 10.5 |
| Temperature (K) | 298(1) | S | 1.2 |
Rexp = 100 [(N-P+C) /Σw (y2obs)]1/2 N-P+C degrees of freedom S = [Rwp/Rexp] Rp = 100 Σ|yobs - ycal| / Σ|yobs| RB = 100 Σk |Ik - Ick | / Σk |Ik Rwp = 100[Σw |yobs - ycal|2 / Σw|yobs|2]1/2
Table III Unit cell, atomic coordinates, isotropic temperature factor and selected geometric parameters (Å, °) for Cu2NiGeS4. Bond valence sum results are shown.
| Atom | Ox. | BVS | Wyck | x | y | z | Foc | B (Å2) |
| Cu | +2 | 1.13 | 4d | 0 | ½ | ¼ | 1 | 0.5(5) |
| Ni | +3 | 1.83 | 2a | 0 | 0 | 0 | 1 | 0.5(5) |
| Ge | +4 | 3.81 | 2b | 0 | 0 | ½ | 1 | 0.5(5) |
| S | -2 | 1.98 | 8i | 0.2574(5) | 0.2574(5) | 0.1216(4) | 1 | 0.5(5) |
| Cu - S | 2.326(3) | Ni - S | 2.330(3) | Ge - Si | 2.238(3) | |||
| Sii - Cu - Siii | 108.6(1) | x4 | S - Ni - Sv | 113.0(1) | x4 | Si - Ge - Svii | 109.9(1) | x4 |
| Sii - Cu - Siv | 109.9(1) | x2 | S - Ni- Svi | 107.7(1) | x2 | Sviii - Ge - Sviii | 109.3(1) | x2 |
Symmetry codes: (i) 0.5 - x, 0.5 - y, 0.5 + z; (ii) y, x, z; (iii) 0.5 - x, 0.5 + y, 0.5 - z; (iv) -y, 1 - x, z; (v) -x, -y, z; (vi) y, -x, -z; (vii) 0.5 - y, - 0.5 + x, 0.5 -z; (viii) -0:5 + x, -0.5+y, 0.5+z. Bond valence sum (BVS): Vi = Σj exp[(Ro - Rij)/b], b = 0:37 Å, r0(Cu-S) = 1.86 Å, r0(Ni-S) = 2.04 Å, r0(Ge-S) = 2.22 Å.
The quaternary alloy Cu2NiGeS4 has a normal valence adamantane-structure and can be described as derivative of the sphalerite structure [2]. As expected for adamantane structure compounds each anion is coordinated by four cations (two Cu, one Ni and one Ge) located at the corners of a slightly distorted tetrahedron. Cu, Ni and Ge cations are equally coordinated by four anions. Figure 2 shows the unit cell diagram for this alloy and is possible observe the tetrahedral coordination around the cations and anions.
All the bond angles in this structure, which vary from 107.7(1)° to 113.0(1)°, are close to the ideal tetrahedral bond angles. An important structural characteristic is the parameter of tetragonal lattice distortion, which is determined as a deviation of the ratio η = c/2a (a and c are unit-cell parameters) from unity [41]. The value of η shows in Table I, close to unity, is indicative of small lattice distortions in the sample synthesized.
The tetrahedrons containing the Ge atoms [mean S…S distance 3.6900(4) Å] are slightly smaller than those containing the Cu atoms [means S…S distance 3.7752(4) Å] and Ni atoms [mean S…S distance 3.854(4) Å] respectively. The interatomic distances are shorter than the sum of the respective ionic radii for structures tetrahedrally bonded [42]. The Cu-S, Ni-S and Ge-S bond distances are in good agreement with those observed in other adamantane structure compounds found in the ICSD database [43]. The structural model was checked by analysis of the interatomic distances using the Bond Valence Sum (BVS) formula based on bond-strength examination [44,45]. These results are shown in Table III, and are close with the expected formal oxidation state of Cu1+, Ni2+, Ge4+ and Se2- ions.
4. Conclusions
The crystal structure of the semiconductor alloy Cu2NiGeS4 was determined using X-ray powder diffraction. This material crystallizes in the tetragonal space group I42m, with a stannite structure. This is a new compound of the I2-II-IV-VI4 family that crystallizes in a sphalerite derivative structure. The improved X-ray powder diffraction data will be submitted to the Powder Diffraction File of the International Centre for Diffraction Data.











 nueva página del texto (beta)
nueva página del texto (beta)