1. Introduction
The base pressure achieved prior to the sputtering deposition of a given material is an important parameter that strongly influences its final characteristics and properties [1-4]. The different vacuum levels achieved before the deposition process is especially important for transparent conductive oxides (TCOs) [5-10], where it is necessary a high transparency to the solar radiation (particularly to the radiation of interest for the device) and a very good conductivity to act as a front electrical contact for application in solar cells [11]. In this context, in a previous work [10] we studied the effect of the variation of this real deposition parameter on the electrical, optical, structural, and chemical characteristics and properties of thin films of Al-doped ZnO [ZnO:Al thin films, commonly known as aluminum-zinc oxide (AZO)], a material that is considered a good substitute of Sn-doped In2O3 [In2O3:Sn, commonly known as indium-tin oxide (ITO)].
The variation tendency of the relevant parameters studied in such a research is summarized in Fig. 1, which was divided into color zones according to the approximate appearance of the corresponding produced AZO thin films. We can observe there the existence of three main vacuum zones, each of which produced thin films that exhibited similar characteristics and properties. The first evidence of the existence of these three vacuum zones is the color of the deposited films, which is clearly different for each vacuum level, as shown in Fig. 2.
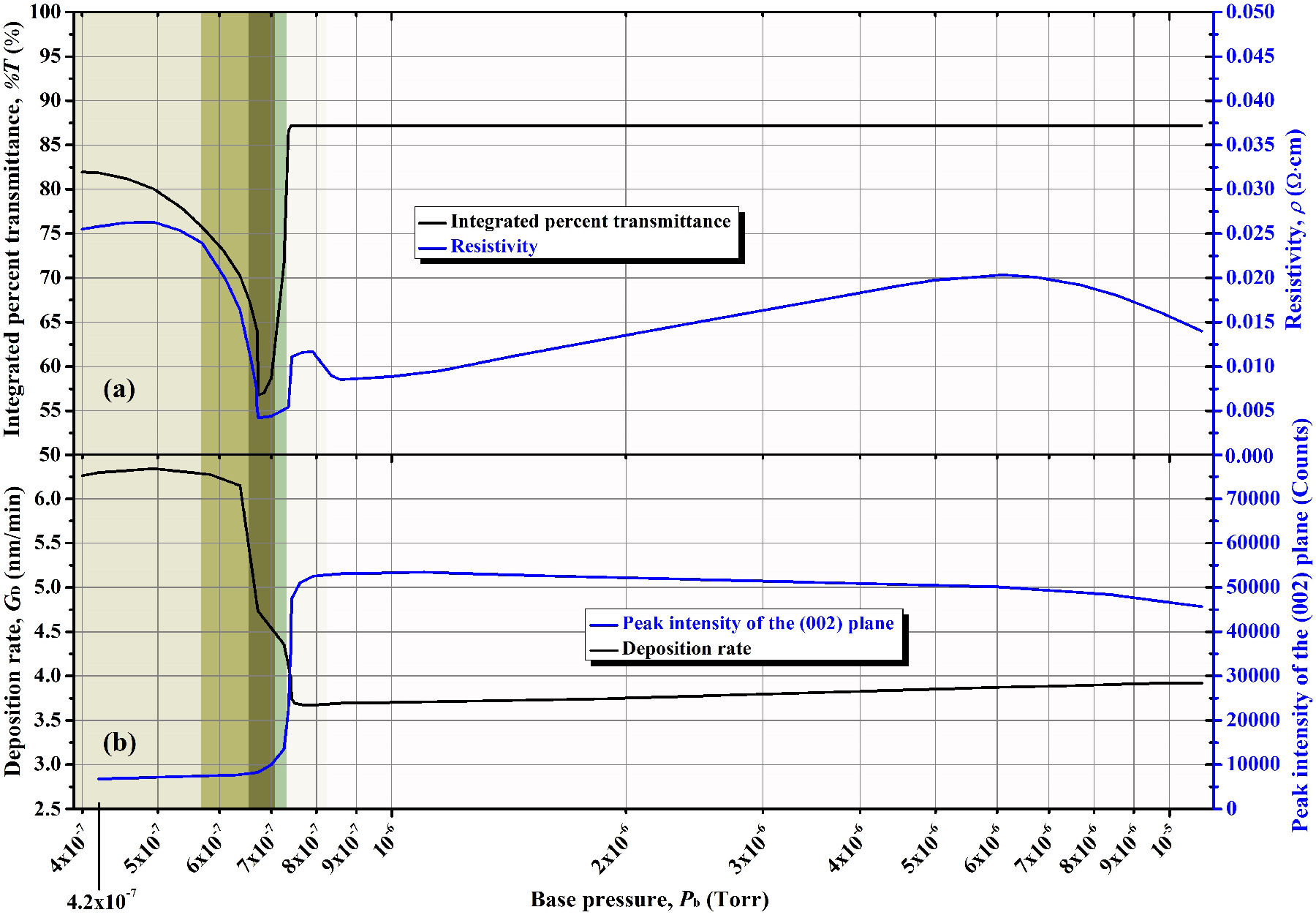
Figure 1 Variation tendency of the (a) integrated percent transmittance and resistivity, and (b) peak intensity of the (002) plane and deposition rate of the AZO thin films as functions of the base pressure achieved before the sputtering- deposition. See our previous work about the effect of base pressure [10].

Figure 2 Digital photographs of the AZO thin films deposited after achieving
representative base pressure values for each vacuum zone: (a) low base
pressure zone [dark yellow zone: from
Thus, values in the highest base pressure zone [
From the previous results, we concluded that the presence of residual gases, at certain level, is beneficial for the properties of sputtering-deposited AZO thin films. In other words, good vacuum levels are not really necessary for TCOs, which is advantageous for the experimenter in saving costs of pumping systems and time in pumping process. Nevertheless, there is an increasingly tendency in the acquisition of better sputtering equipment with excellent pumping systems, thus allowing experimenters the opportunity to reach very good vacuum levels. However, as mentioned in the previous paragraphs, the best vacuum levels achieved by us lead to dark yellow and resistive AZO thin films (see Fig. 1 and Fig. 2), which are undesirable characteristics for TCOs.
With the above in mind, the present work aims to improve the transmittance of the AZO
films deposited at room temperature after achieving a constant and very good vacuum
level. Also, the deposition process for improving transmittance should not impact
negatively on the electrical properties. To simplify the process, postdeposition
treatments to improve transmittance are discarded. In this way, we studied the
effect of the deposition parameters usually employed in the sputtering technique on
the main properties of AZO thin films when a very good vacuum pressure is achieved
prior to the sputtering-deposition:
As a main result, we observed that the control of argon pressure, without the addition of oxygen, is better for providing a good compromise between transmittance and resistivity of the AZO samples deposited when a high vacuum level is achieved. Furthermore, an interesting correlation was found between the optical and electrical properties and the chemical composition of the AZO films, the latter depending on the argon pressure. Also, the inverse relationship between crystallinity and deposition rate was confirmed, in which deposition rate inversely depends on argon pressure. The overall results presented in this paper complement our previous work about the effect of base pressure on properties of AZO thin films [10], and therefore the reader is referred to such a work for more details.
2. Experimental details
2.1. Deposition methodology
The deposition equipment used for the present work was a commercial ATC-ORION 8
HV sputtering system from AJA International, Inc. It consisted of a 34.7 cm
diameter and 39.8 cm height chamber with three different magnetron guns in a
confocal geometry. In this configuration, the magnetron sputter guns, which can
host targets of 7.62 cm (3 inch) in diameter, are tilted 3
The AZO thin films were deposited onto
The distance from the center of the target to the center of the substrate was
fixed at 18 cm, and a substrate rotation of 13 rpm was used to achieve good
homogeneity during the material deposition. The films were deposited at room
temperature with a DC power of 120 W, which corresponds, when divided by the
target area (45.60 cm
Table I Flow rates of oxygen and argon employed for studying the effect of oxygen volume percent in the argon/oxygen mixture, by maintaining a total working pressure of 4.0 mTorr (0.53 Pa).
| Oxygen volume | Oxygen flow | Argon flow | Total flow |
| percent (%) | rate (sccm) | rate (sccm) | rate (sccm) |
| 0.0 | 0.00 | 19.96 | 19.96 |
| 1.5 | 0.30 | 19.96 | 20.26 |
| 2.0 | 0.40 | 19.60 | 20.00 |
| 2.5 | 0.50 | 19.50 | 20.00 |
In the present study, the base pressure achieved prior to the
sputtering-deposition was around
2.2 Characterization techniques
The thickness of all the deposited AZO thin films was measured with a Dektak 3030 profilometer, for which a simple lift-off technique was used on the films. The deposition rate, which was assumed constant along the film growing process, was calculated by dividing the measured thickness by the deposition time. The sheet resistance of the AZO thin films was measured by using a Jandel RM3 four-point probe system, and the resistivity was calculated by multiplying the measured thickness and the sheet resistance.
For the optical properties, the percent transmittance (%
The structure of the deposited material was analyzed by means of a PANalytical X’Pert PRO MPD Alpha 1 powder X-ray diffractometer. The crystallite size was calculated from the X-ray diffraction (XRD) patterns by using the Scherrer equation [12-14]:
where
For the analysis of the chemical composition of the material, the AZO thin films
were measured by X-ray photoelectron spectroscopy (XPS) by using a PHI
Multitechnique system (from Physical Electronics) with a monochromatic X-ray
source from the AlK
The quantitative analysis of the XPS spectra was done via peak-fitting using the AAnalyzer
The chemical composition analysis, on the other hand, was done using a method that takes into consideration physical parameters, that the films are homogeneous, and that the peak areas are proportional to their respective atomic densities according to the following equation [20,25-28]:
where
3. Results and discussion
If a TCO thin film that is deposited by sputtering under certain conditions results with a low transmittance, the first solution taken into account for the next deposition process is the addition of oxygen in the sputtering chamber. Because the darkening is usually assigned to an excess of metal in the TCO film (which maybe induce intragap states, and hence absorption in the visible spectral range), the addition of oxygen in the sputtering chamber during the deposition process, which oxidizes the material, is always beneficial for transmittance of most of TCO materials. However, the addition of oxygen is detrimental for the electrical properties of the deposited material, because this decreases both the mobility and carrier concentration (see, for example, that reported by Zhu et al. [33] and Shih et al.[34]). In Fig. 3, for our case with the AZO films obtained by using a total deposition pressure of 4.0 mTorr (0.53 Pa), a clear increase of resistivity with the increase of the oxygen volume percent in the mixture of argon/oxygen is observed, which resulted completely detrimental for the case of 2.5%, in which the film is highly resistive. This observation is generally associated with the decrease of both the oxygen vacancies and interstitial zinc due to the incorporation of oxygen in the deposited material as the oxygen content is increased in the plasma discharge[33,34]. Given this result, we decided to probe other parameters to improve transmittance, and thus to find better options than the addition of oxygen in the sputtering chamber.
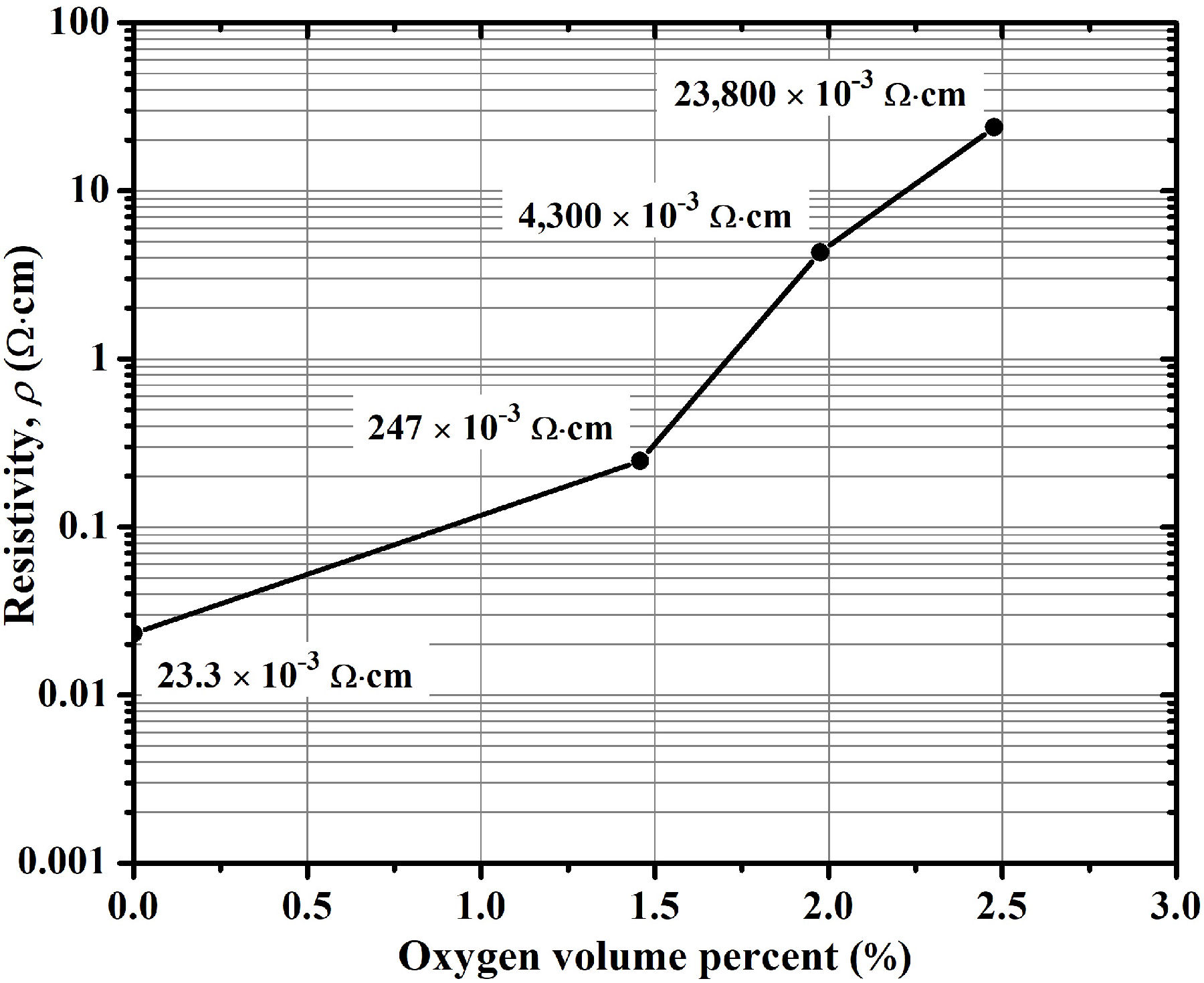
Figure 3 Variation of resistivity of the AZO thin films as a function of oxygen volume percent in the argon/oxygen mixture during the sputtering-deposition.
Thus, as plotted in Fig. 4, we observed that the increase of the argon deposition pressure, without the addition of oxygen, increases the transmittance of the AZO thin films, and although an increase in the value of this deposition parameter also worsens the electrical properties, this occurs in a lesser extent compared to the use of oxygen during the film deposition. (For a better optical comparison, the transmittance spectra of these thin films are shown in Fig. 5). With this experiment, we also observed a clear and abrupt darkening of the AZO thin films when the argon working pressure is lower than 4.0 mTorr (0.53 Pa), although thin films deposited under this condition are more conductive (we indicate this argon pressure zone with a gray area in this and in the following figures). We partially conclude here that the use of higher argon pressure [higher than 4.0 mTorr (0.53 Pa)] under the deposition conditions previously described for this work (Sec. 2.1) is beneficial for the transmittance without greatly impacting on the electrical properties of the AZO thin film when good vacuum levels are achieved prior to the sputtering-deposition.
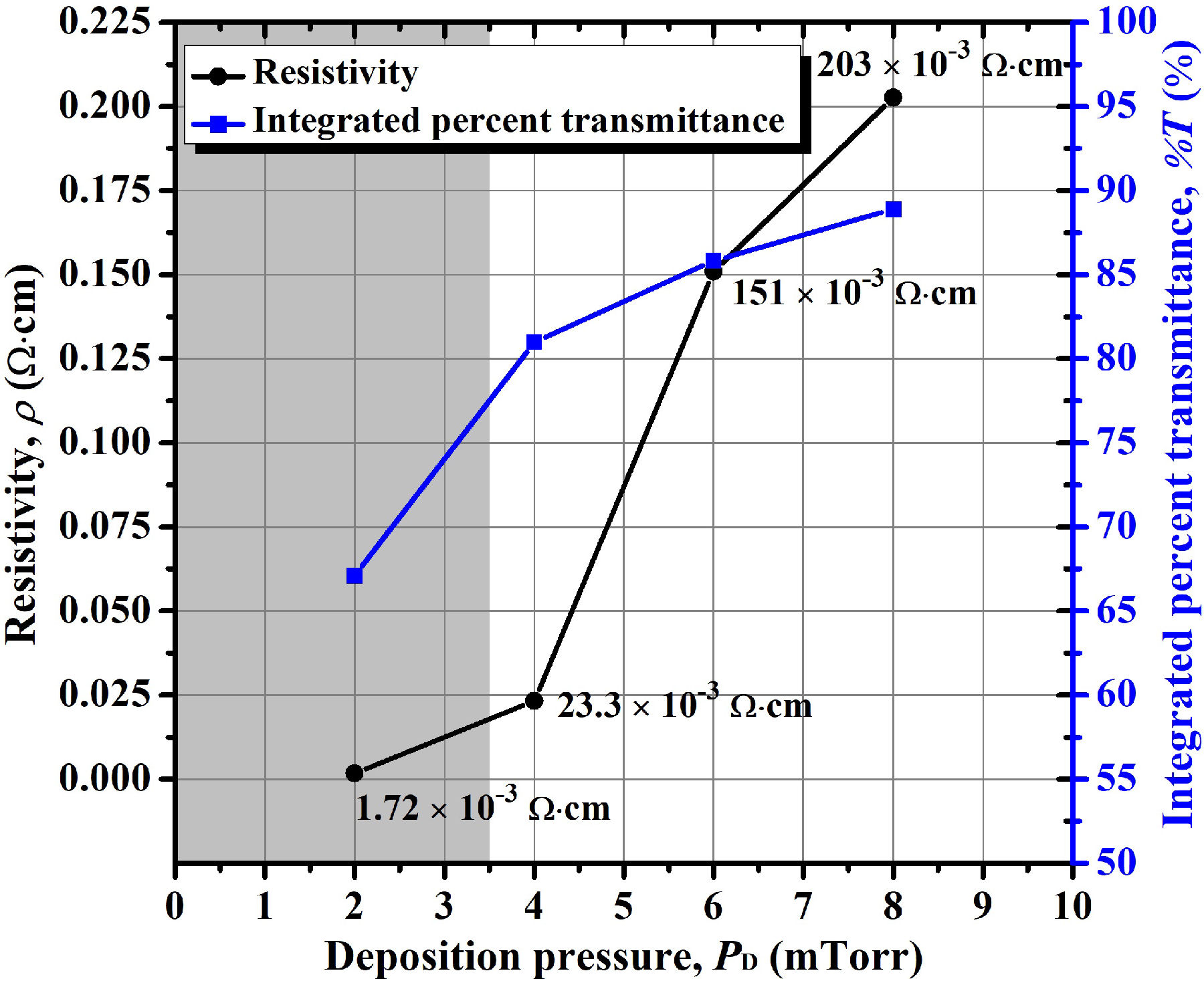
Figure 4 Variation of resistivity and integrated percent transmittance of the AZO thin films as a function of argon deposition pressure.
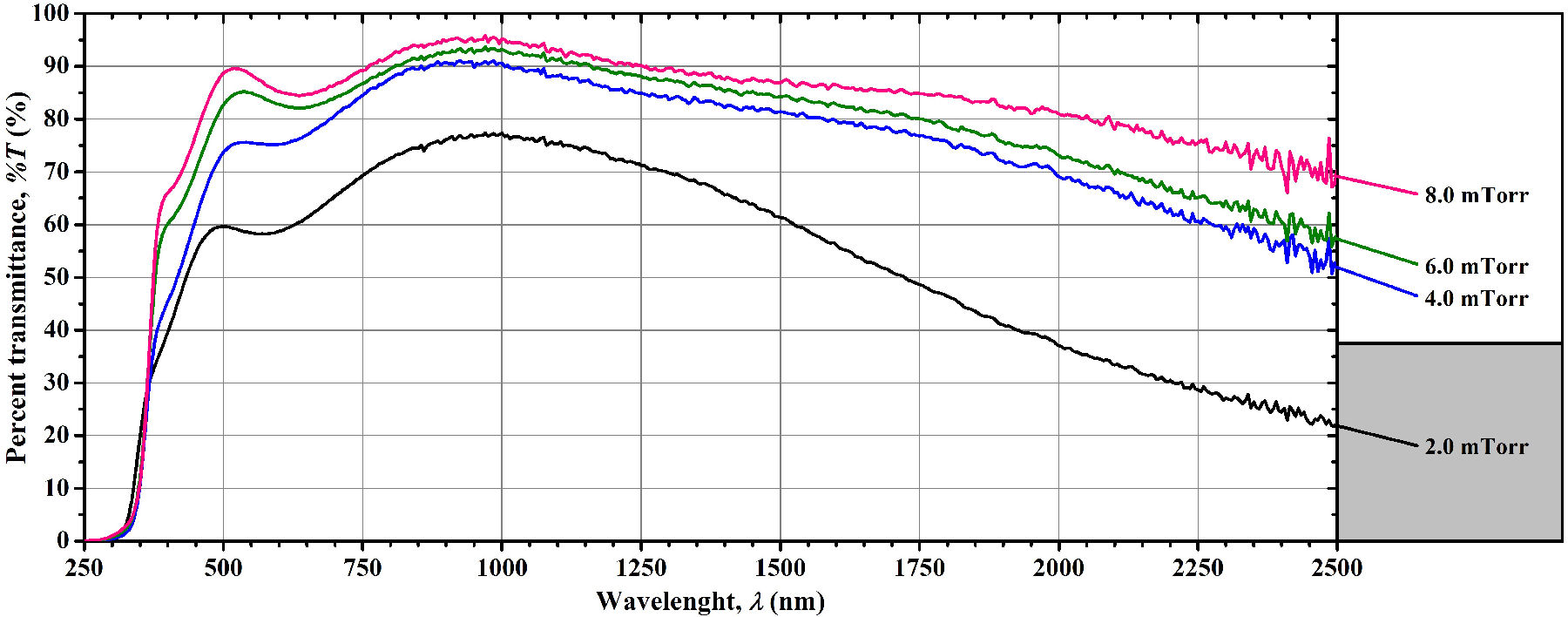
Figure 5 Percent transmittance spectra of the AZO thin films sputtering-deposited at different argon pressure values.
Given that the vacuum level used in this work prior to the film deposition was
constant and very low [good vacuum levels:
To begin, Fig. 6 shows the XRD patterns of the
AZO thin films deposited at different argon pressures. It is observed that all of
the recorded diffractograms exhibit an intense peak at around 34.3°, which
corresponds to the (002) crystalline plane of the ZnO hexagonal wurtzite structure,
according to PDF # 36-1451. This indicates a
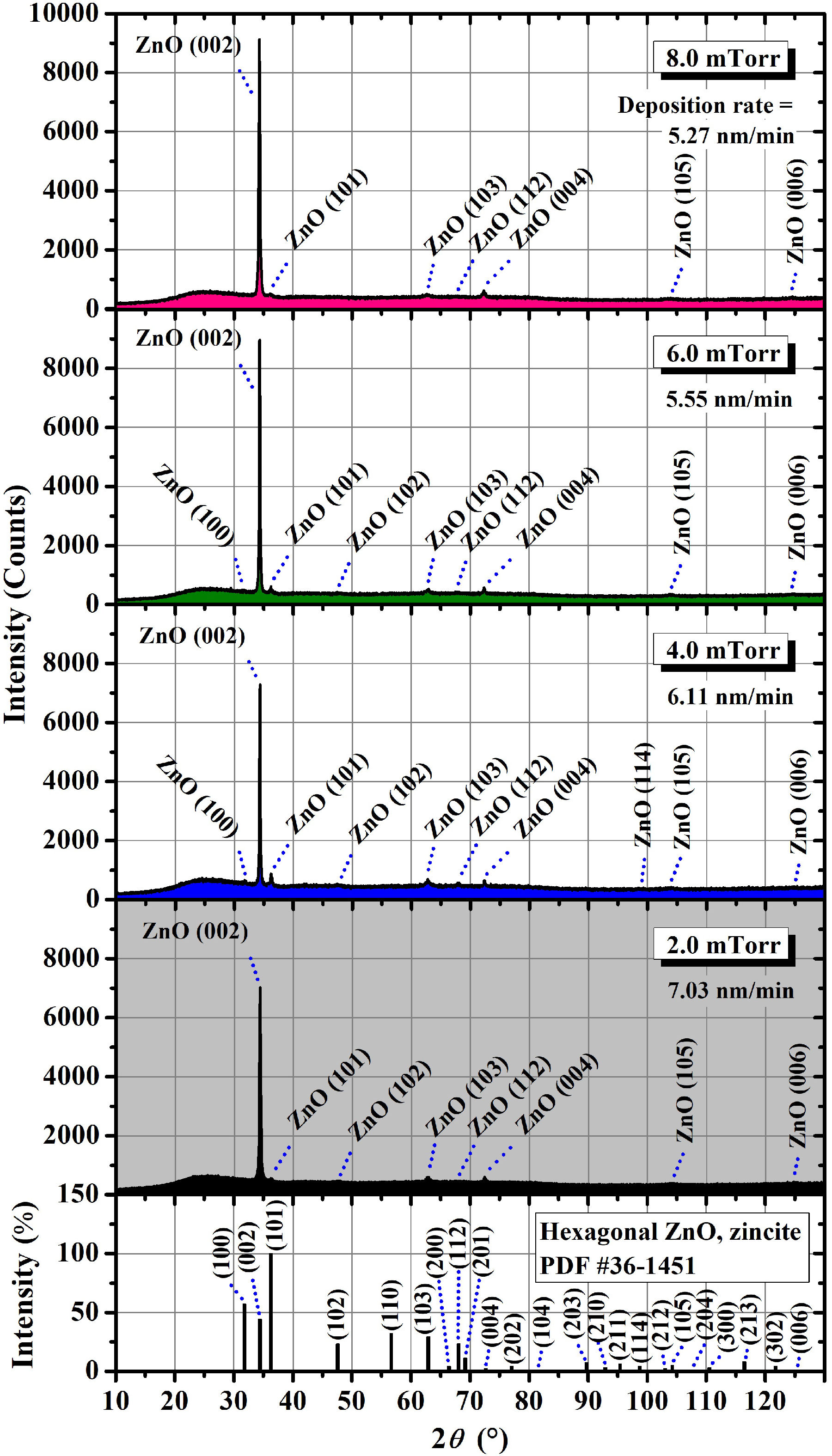
Figure 6 X-ray diffractograms of the AZO thin films sputtering-deposited at different argon pressure values.
The most important of them are located at around 36.3, 62.8, 67.9, and 72.4∘, which correspond, respectively, to the (101), (103), (112), and (004) crystalline planes of the same hexagonal structure. In a rough comparison, all the diffractograms present very similar characteristics. The difference, which will be discussed below, lies in the intensity of the XRD peaks, which can be taken as an approximate indication of the crystallinity of the films.
Figure 7 shows the change of deposition rate and the peak intensity of the (002) crystalline plane obtained from the XRD patterns of the AZO samples sputtering-deposited at different argon pressure values (Fig. 6). A clear diminution of deposition rate with the increase of argon deposition pressure is observed. Furthermore, the intensity of the peak corresponding to the (002) crystalline plane seems to inversely depend on the deposition rate at which the AZO thin films grow. This latter correlation was also observed in our previous work about the effect of the base pressure achieved prior to the sputtering-deposition [10], although in such a case the variation of the peak intensity was enormous due to the obtainment of deposition rates of even between 3 and 4 nm/min, a range that greatly affects the peak intensity of the (002) plane, this according to our previous paper [see Fig. 1(b)]. In such a paper, we reported that the deposition rate was strongly decreased by the presence of residual gases (that is, in bad vacuum levels), whose presence and subsequent dissociation probably exhaust the electron energy in the plasma, which results in a suppressed ionization of argon and, hence, in the decrease of deposition rate (as based on conclusions by Wang et al. [35] about the effect of water vapor on ITO films). For the present case, where the base pressure is constant, the phenomenon affecting deposition rate is distinct. Here, the increase of argon pressure increases the number of particles in the plasma discharge and thus increases the probability of collision, resulting in a reductionn ofn then mean free path and therefore in a diminution of deposition rate, thus increasing the peak intensity of the (002) crystalline plane.
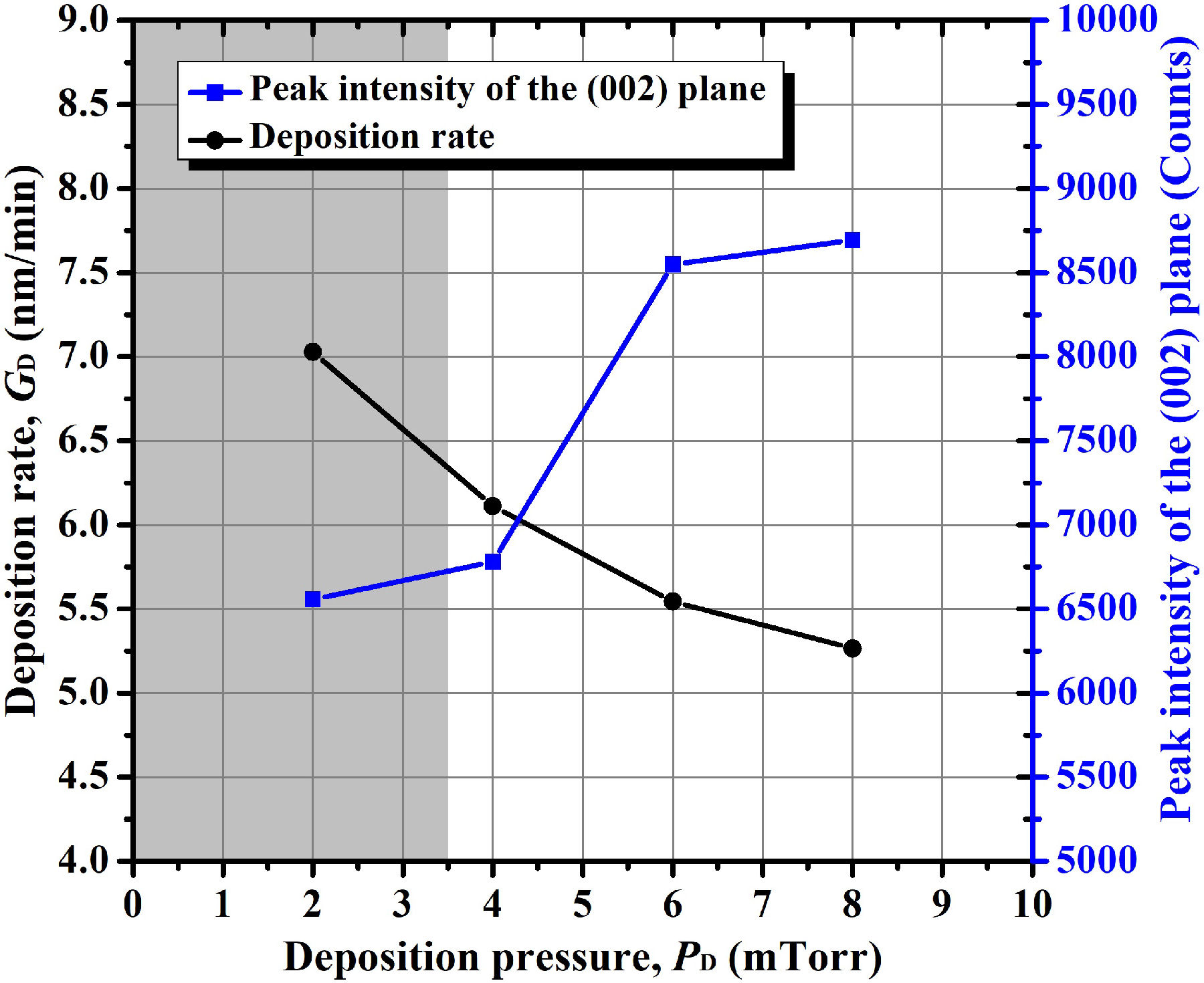
Figure 7 Variation of deposition rate and peak intensity of the (002) crystalline plane of the AZO thin films as a function of argon deposition pressure.
It is important to note that the crystallinity showed by these AZO thin films
deposited under different argon pressure values when a constant and very low base
pressure is achieved [
However, before discussing the chemical analysis, it is necessary to discard the parameters related with the structure of the AZO thin films as transmittance-determining factors. Thus, we also measured the crystallite size of the samples deposited by varying the argon pressure. For this, the Scherrer equation [Eq. (1)] was applied using the peak of the (002) plane. As a result, in Fig. 8 we plotted the variation of crystallite size with deposition pressure, in which it is observed that the variation of crystallite size is not very large (for the deposition pressure interval studied), and therefore it cannot be associated with the transmittance of the films. In fact, the magnitude of crystallite size seems not to be strongly related lated with other important parameters, like resistivity (as also shown shown in Fig. 8). Thus, as partial conclusions, when varying the argon pressure (in the interval studied), the obtained crystallite size does not largely define the optical and electrical properties.
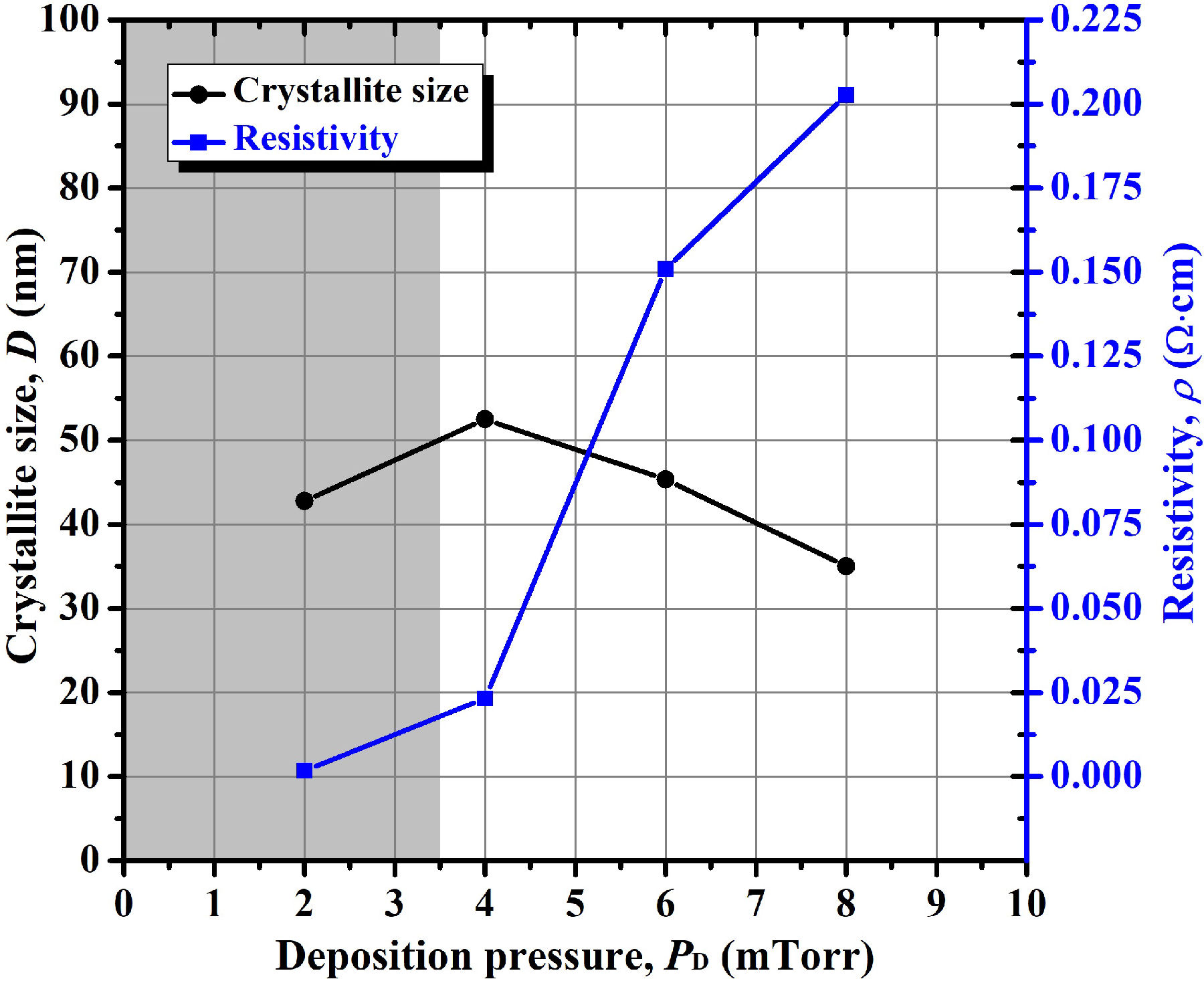
Figure 8 Variation of crystallite size and resistivity of the AZO thin films as a function of argon deposition pressure.
At this point, it is now convenient to address the chemical analysis. To begin, the
resolved high-resolution XPS spectra of the AZO thin films deposited at different
argon pressures are shown in Fig. 9, and the
positions for each determined component are presented in Table II. The Zn
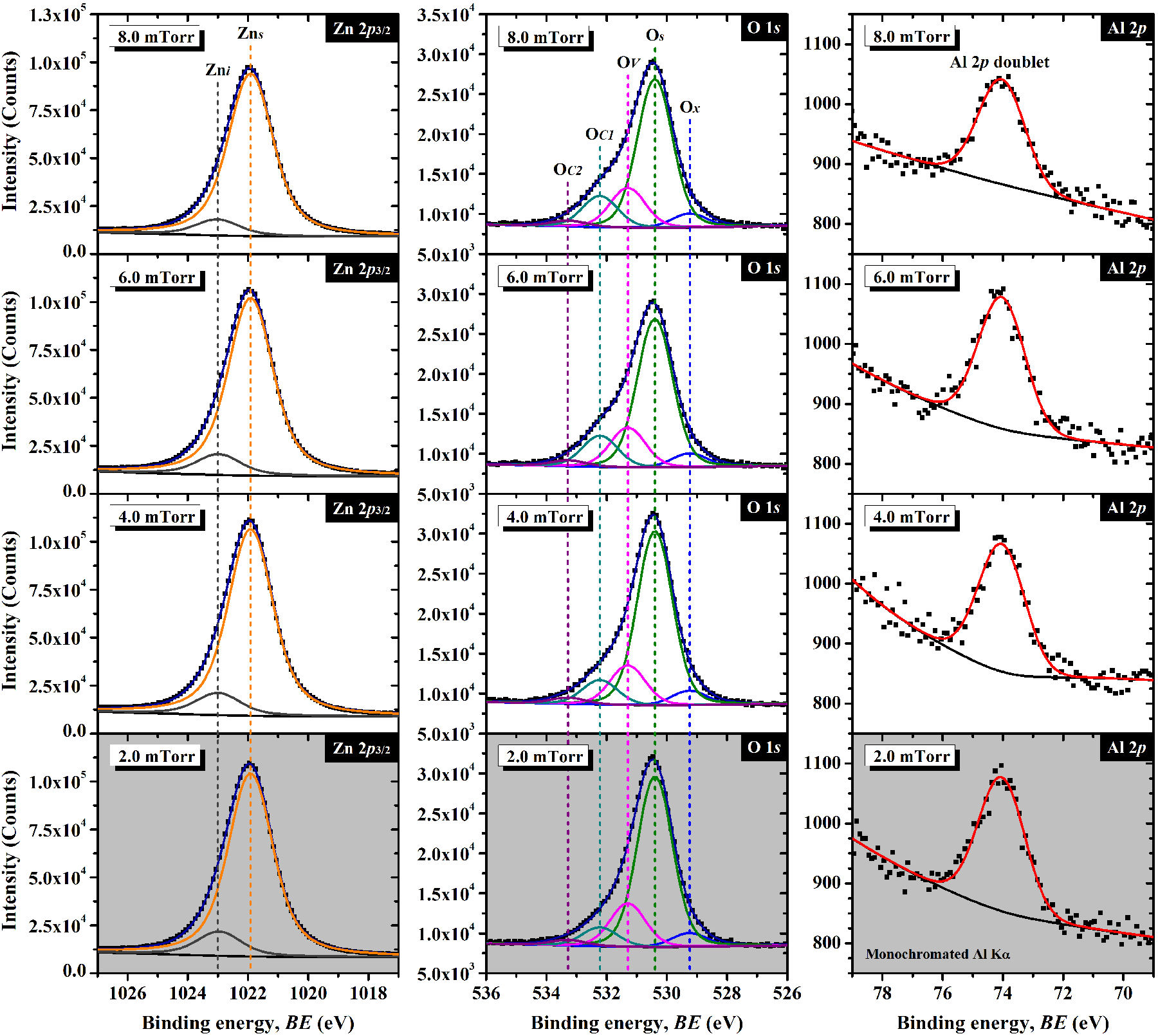
Figure 9 Resolved high-resolution photoemission spectra of the AZO thin films sputtering-deposited at different argon pressure values.
As expected and mentioned in the previous paragraphs, the explanation for the
variation of optical and electrical properties of AZO films deposited at different
argon pressures was found after the chemical analysis of the samples. The results of
this chemical analysis, as calculated from Eq. (2), are presented in Fig. 10. Here, we observed a dependence of the
chemical composition, especially in terms of structural defects, with the change of
argon pressure. For the discussion of data plotted in this figure, it is convenient
to begin with the aluminum case, whose variation with deposition pressure does not
seem to show a significant change, so its variation can be considered negligible,
which simplifies the interpretation of the results. Therefore, the electrical and
even the optical properties of the AZO material were affected mainly by the change
of zinc and oxygen components as the deposition pressure is varied. Regarding to
these components, it is also interesting to observe that the chemical composition
assessment of peaks Zn
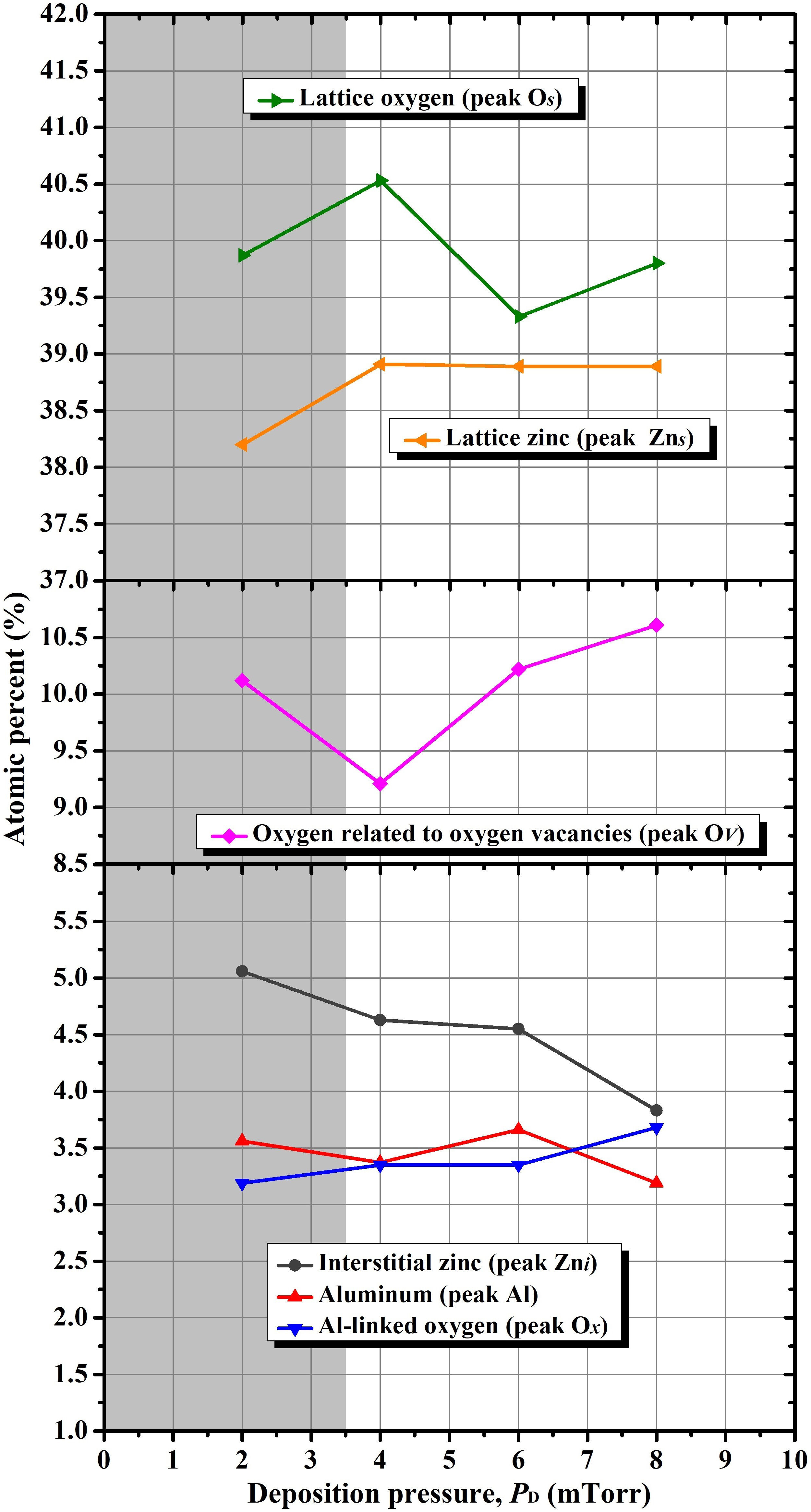
Figure 10 Variation of the chemical composition of the AZO thin films as a function of argon deposition pressure.
A notable observation in Fig. 10 is the
variation of the interstitial zinc content with increasing deposition pressure; the
interstitial zinc content (represented by the peak Zn
With this study, we found that the variation of deposition pressure, with only argon as working gas, affects the optical and electrical properties of the AZO thin films deposited in low content of residual gases. The use of deposition pressure equal and higher than 4.0 mTorr (0.53 Pa) under the conditions described in Sec. 2.1 leads to transparent and moderately conductive AZO thin films. Furthermore, the impact on the electrical properties is lesser when increasing the argon pressure than when introducing oxygen in the sputtering chamber. Also, an interesting explanation of the results was done based on the chemical composition analysis.
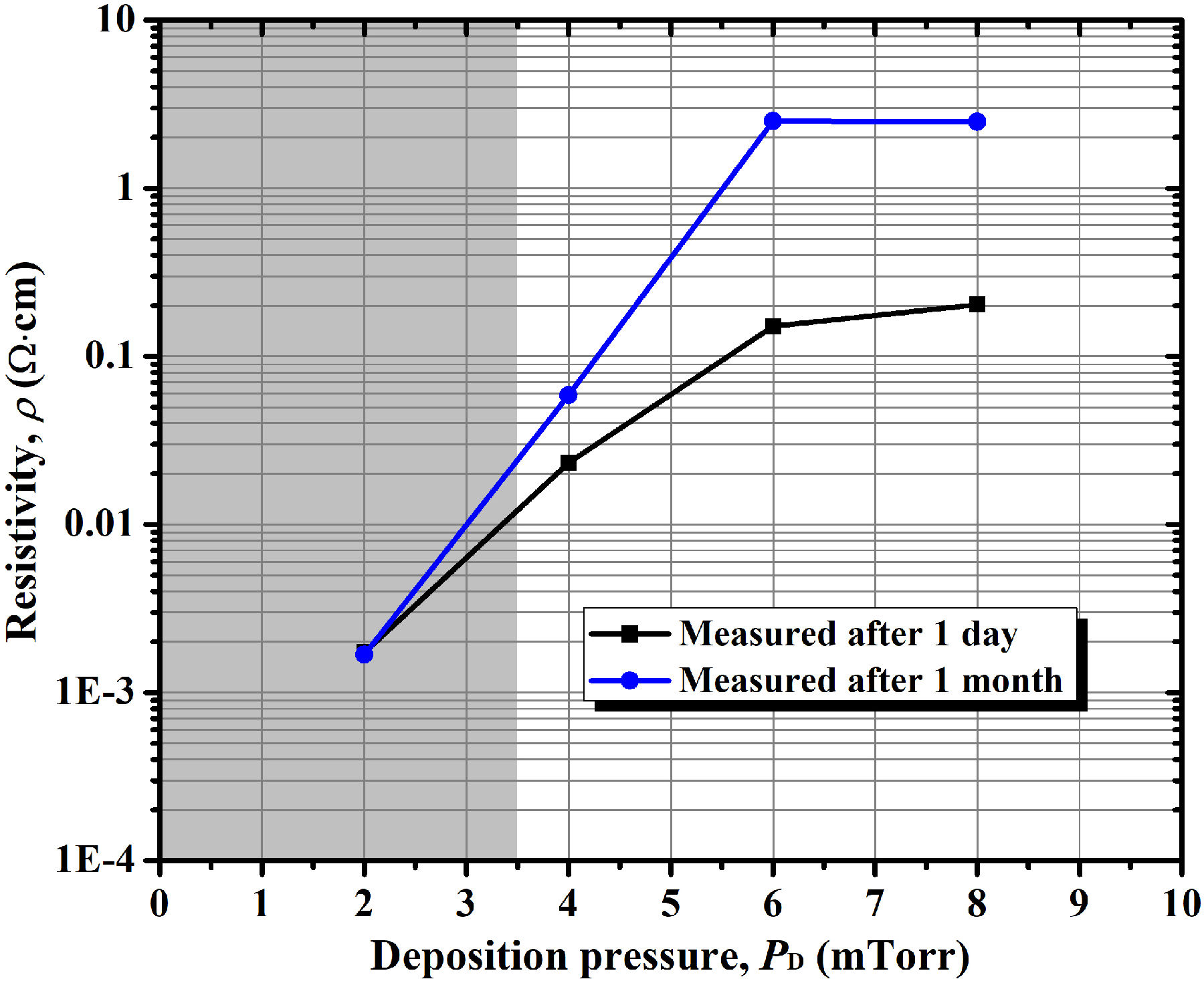
Finally, we want to include in this paper an additional preliminary experiment performed on the discussed AZO thin films with the aim of finding a rough indication of their stability to the environment. For this, we measured the resistivity of the AZO thin films again after one month of aging in the ambient air (under uncontrolled humidity and light). The resistivity of the films measured one day and one month after their deposition are plotted in Fig. 11. We observed here that thin films deposited at higher argon pressure do not only show higher resistivity, but also show more instability to the environment. Among the deposited films, the dark gray AZO thin film deposited at 2.0 mTorr (0.27 Pa) shows no change in resistivity, which indicates a high stability to the environment. The instability to the environment of the films deposited at high deposition pressures may be related to the neutralization of oxygen vacancies by oxygen provided by the environment during the days. In contrast, the film deposited at low deposition pressure (the dark zone) shows excellent stability probably due to the relatively high content of zinc interstitials that strain the lattice restricting the incorporation of ambient oxygen.
4. Conclusions
We deposited by DC magnetron sputtering
On the other hand, the increase in argon total pressure, without the addition of
oxygen, also increased both the transmittance and resistivity, although the increase
of the latter occurred in a lesser extent compared to the addition of oxygen. For
example, an integrated %T of 88.9% and a resistivity of 203 m Ω∙cm
were obtained by increasing the deposition pressure up to 8.0 mTorr (1.07 Pa). On
the contrary case, the AZO thin film resulted in a dark gray color (%
The change of resistivity and transmittance was associated with the chemical
composition of the AZO material: the lower the deposition pressure, the higher the
interstitial zinc content in the AZO thin film. The increase in the content of
interstitial zinc, alongside with oxygen vacancies, conferred to the films good
electrical properties but poor transmittance. On the other hand, the aluminum
content was almost constant (
Furthermore, we observed that the increase in deposition pressure decreased the deposition rate at which the AZO thin films grow. Also, with the decrease of deposition rate the peak intensity of the (002) crystalline plane was slightly increased (as compared with the effect of the base pressure). However, in the present study, we did not find a strong direct influence of these parameters on the optical and electrical properties, although the increase of the interstitial zinc content at low deposition pressure is probably related with the observed slightly decrease of crystallinity.











 nueva página del texto (beta)
nueva página del texto (beta)