1. Introduction
The addition of a II-VI binary to chalcopyrite I-III-VI
These types of materials have received considerable attention mainly because they can
be useful for their potential applications in the fabrication of low cost solar
cells and their large magneto-optical effects, which are observed when II are
paramagnetic atoms [10,11]. We are currently investigating the synthesis and
structural characterization of chalcogenide diamond-like families [12-16],
and as part of ongoing studies, in this work we report the synthesis and a
crystallographic characterization of the new chalcogenide quaternary compound CuVInSe
2. Experimental
2.1. Preparation of the sample
Polycrystalline sample was synthesized using the melt and annealing technique.
Stoichiometric quantities of the elements with purity of 99.99% were charged in
a synthetic silica glass ampoule, which was previously subjected to pyrolysis in
order to avoid reaction of the starting materials with silica glass. Then, the
ampoule was sealed under vacuum (
2.2. Scanning electron microscopy
The stoichiometric relation of the samples was investigated by Scanning Electron
Microscopy (SEM) technique, using a FE-8 SEM, Jeol 6301-F equipment. The
micro-chemical composition was found using an energy dispersive X-ray
spectrometer (EDS) coupled with a computer-based multichannel analyzer (MCA),
(Delta III analysis and Quantex software, Kevex). For the EDS analysis K
2.3. Differential thermal analysis
The differential thermal analysis (DTA) was carried out in a fully automatic
Perkin-Elmer apparatus with Pt/Pt-Rh thermocouples. Au or Ag was used as
internal standards, according to the expected melting point of the sample. The
heating and cooling rates were controlled to 20 K/h. Transition temperatures
were manually obtained from the
2.4. X-ray powder diffraction
A small amount of the sample was ground in an agate mortar and pestle and mounted
on a flat zero-background holder covered with a thin layer of petroleum jelly. A
Siemens D5005 powder diffractometer was used for data collection with the
conditions given in Table I. The X-ray
Power Difraction (XRPD) data was collected at 293(1) K, in
TABLE I Experimental parameters for data collection of the CuVInSe3 compound.
| diffractometer | Siemens D5005 | 2θ range | 10-100° |
| radiation | CuKα | step size | 0.02° (2θ) |
| instrumental settings | 40 kV, 30 mA | counting time | 40 s |
| wavelenght | λ = 1:54056 Å | specimen rotation | 15 r.p.m. |
| monocrhomator | graphite | external standard | quartz |
| scan mode | θ/2θ (reflection) | temperature | 298(1) K |
3. Results and Discussion
3.1. Scanning Electron Microscopy
The obtained ingot (15 mms long and 8 mms diameter) was homogeneous at sight, with no voids in the surface and light gray color. As it is routine in our laboratory, stoichiometry and homogeneity of the ingots are tested using scanning electron microscopy (SEM); for that, a slice 1 mm thick was cut from the center of the ingot. In Fig. 1, the results of SEM measurements are shown. It When measured, a little excess of selenium was found in the points located in the inner part of the slice whereas for the nearest points to the external surface of the ingot the selenium is in defect. This behavior may be due to the long period of annealing (30 days) for which little amounts of selenium in the surface of the ingot may go out. For Se, the measured values are ∼3% higher that the estimated experimental error (±5%); for cations Cu, In and V, the measured values are in agreement with nominal in the range of the experimental error.
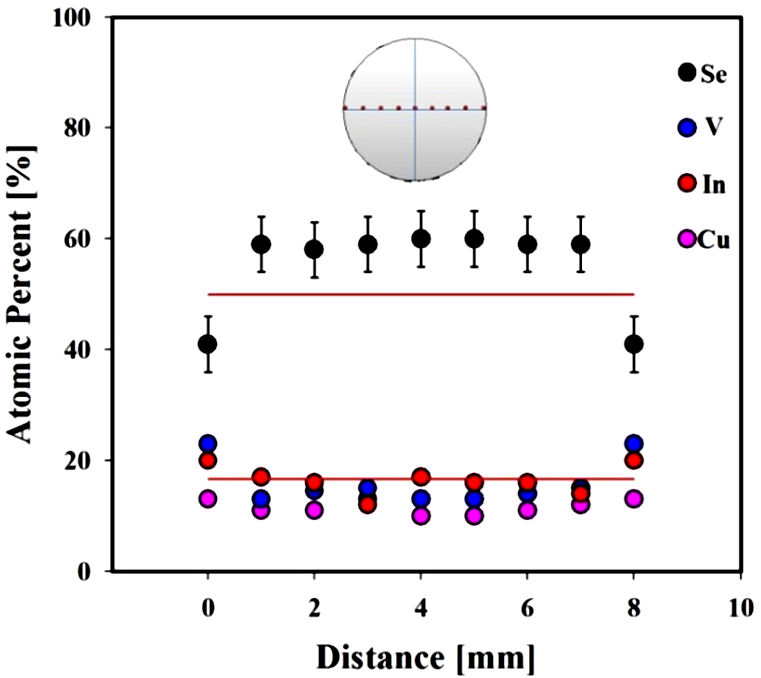
Figure 1 SEM measurements for CuVInSe
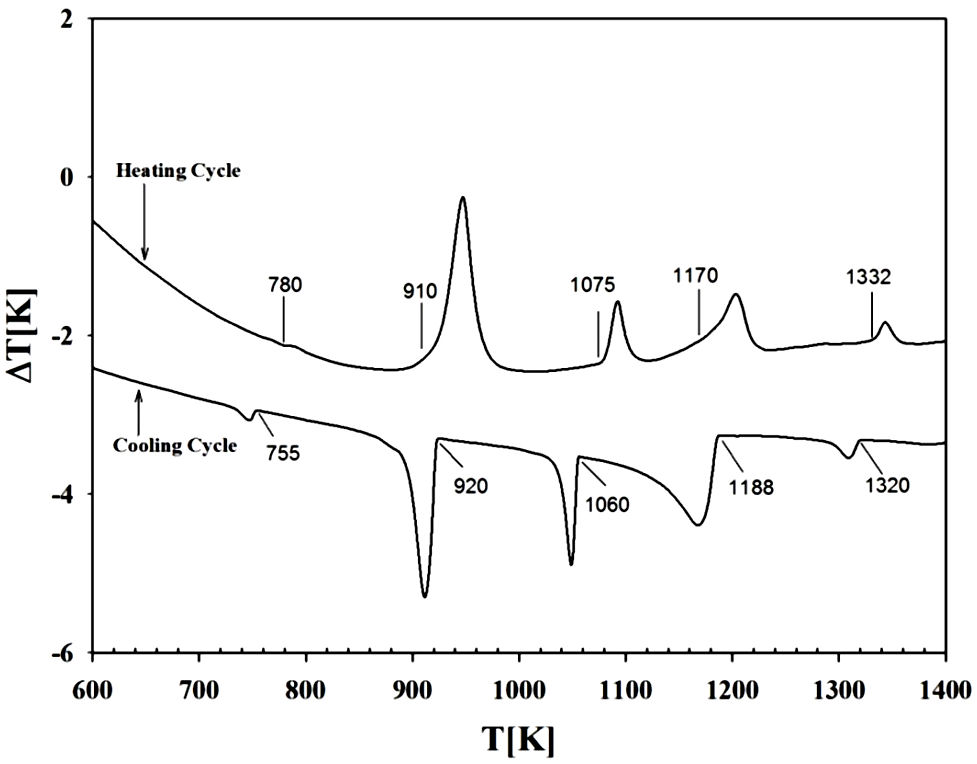
3.2. Differential thermal analysis (DTA)
DTA runs were carried out on the sample as indicated above. The transition
temperatures as well as the type of melting were obtained from the peaks on the
DTA heating and cooling curves. Each transition temperature was determined from
the base intercept of the tangent to the leading edge of the peak in the
differential signal (see Fig. 2). It can be
observed that CuVInSe
3.3. X-ray powder diffraction analysis
The X-ray powder pattern of CuVInSe
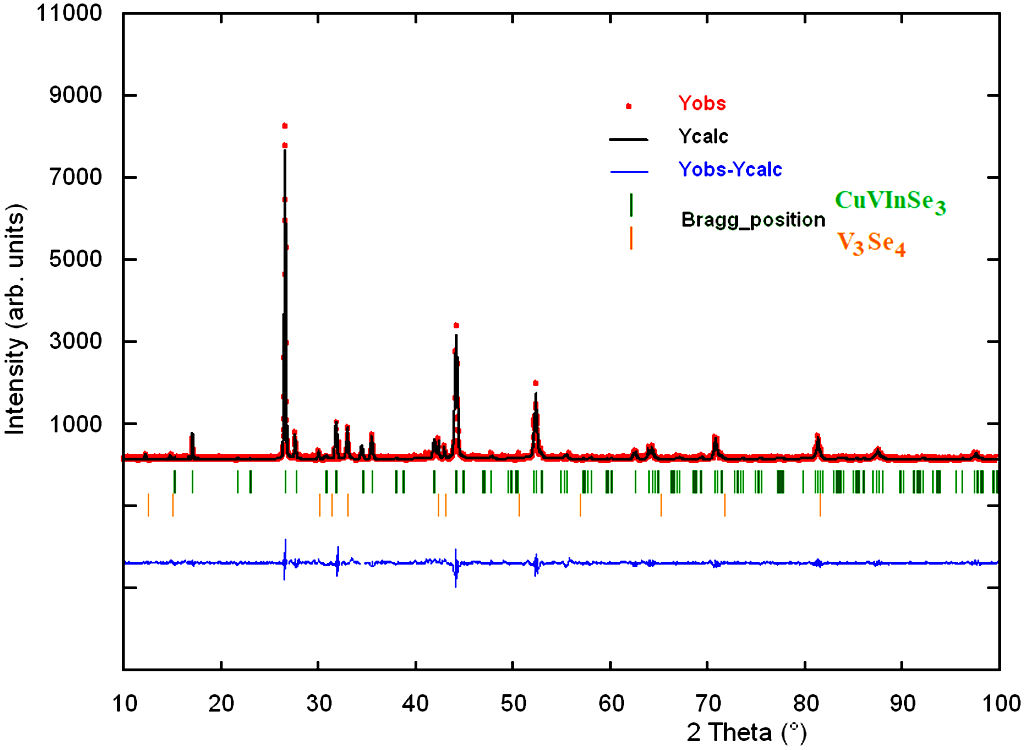
Figure 3 Final Rietveld plot showing the observed calculated and
difference pattern for the CuVInSe
The Rietveld [19] refinement was performed
using the Fullprof program [20]. The
indexed unit cell results were taken as starting parameters. Atomic positions of V
TABLE II Results of Rietveld refinement for CuVInSe3
| Molecular formula | CuVInSe |
D |
5.29 (g/cm |
| Molecular weight | 1243.0 (g/mol) | N |
4001 |
| Crystal system | Orthorhombic | N |
153 |
| Space group | P |
Peak-shape profile | Pseudo-Voigt |
| Z | 2.667 (8/3) | Rexp | 6.6 % |
| a | 5.7909(4) | Rp | 8.7 % |
| c | 11.625(1) | Rwp | 8.8 % |
| V | 389.84(5) | S | 1.3 |
Figure 3 shows the observed, calculated and difference profile for the final cycle of Rietveld refinement. The lower trace is the difference curve between observed and calculated patterns. The Bragg reflections are indicated by vertical bars. Unit cell parameters, atomic coordinates and isotropic temperature factor are shown in Table III.
TABLE III Unit cell, atomic coordinates, isotropic temperature factors for CuVInSe3, derived from the Rietveld refinement M = (Cu1+V1+In1)
| Space group P | |||||||
| Atom | Ox. | Site | x | y | z | foc | B (Å2) |
| Cu | +1 | 2e | 0 | 0 | 0 | 1 | 0.4(4) |
| V | +2 | 2d | 0 | 1/2 | 1/4 | 1 | 0.4(4) |
| In | +3 | 2b | 1/2 | 0 | 1/4 | 1 | 0.4(4) |
| 2f | 1/2 | 1/2 | 0 | 1/3 | 0.4(4) | ||
| M | 2f | 1/2 | 1/2 | 0 | 1/3 | 0.4(4) | |
| 2f | 1/2 | 1/2 | 0 | 1/3 | 0.4(4) | ||
| Se | -2 | 8n | 0.2553(5) | 0.2572(5) | 0.1231(3) | 1 | 0.4(4) |
Table IV shows the distance lengths and
bond angles for CuVInSe
TABLE IV Distance lengths (Å) and bond angles (°) for CuVInSe3.
| Cu-Se | 2.518(3) | V-Se | 2.540(3) | In-Se | 2.530(3) | M-Se | 2.456(3) |
| Seiii-Cu-Seiv | 108.1(1) | Seiii-Cu-Sev | 112.1(1) | Seiii-Cu-Se | 108.3(1) | ||
| Seiv-Cu-Sev | 108.3(1) | Seiv-Cu-Se | 112.1(1) | Sev-Cu-Se | 108.1(1) | ||
| Se-V-Sexii | 108.5(1) | Sex-V-Sexi | 108.5(1) | Sex-V-Se | 108.5(1) | ||
| Sex-V-Sexii | 111.4(1) | Sexi-V-Se | 111.4(1) | Sexi-V-Sexii | 108.5(1) | ||
| Se-In-Sei | 107.9(1) | Se-In-Seviii | 111.9(1) | Se-In-Seix | 108.7(1) | ||
| Seviii-In-Sei | 108.7(1) | Seviii-In-Seix | 107.9(1) | Sei-In-Seix | 111.9(1) | ||
An important structural characteristic is the parameter of tetragonal lattice
distortion, which is determined as a deviation of the ratio
CuVInSe
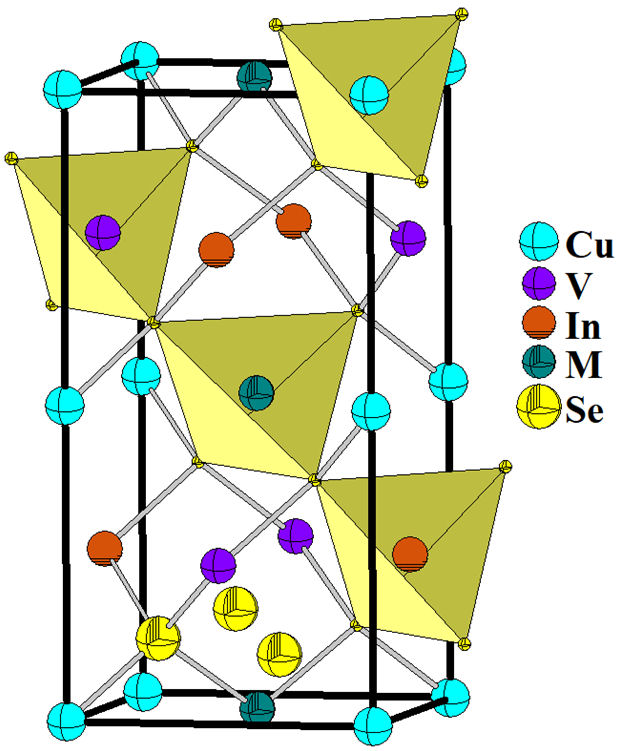
Figure 4 Unit cell diagram for the quaternary chalcogenide CuVInSe
The interatomic distances are shorter than the sum of the respective ionic radii
for structures tetrahedrally bonded[26].
The bond distances Cu-Se [2.518(3) Å], V-Se [2.540(3) Å] and In-Se [2.530(3) Å]
agree well with those observed in other adamantane compounds such as CuInSe
4. Conclusions
A new quaternary chalcogenide has been synthetized and structurally characterized.
The DTA indicates that this compound melts at 1332 K. The crystals of
CuVInSe3 belong to the tetragonal system with space group P











 nova página do texto(beta)
nova página do texto(beta)