PACS: 61.05.cp; 61.50.Nw; 61.66.Fn; 61.40.b
1. Introduction
The family of quaternary I2-II-IV-VI4 compounds, where I = Cu or Ag; II=Zn, Cd, Mn, Fe, or Ni; IV=Si, Ge, or Sn; and VI = S, Se, or Te; obtained from the tetrahedrally coordinated derivatives of the II-VI binaries1, have received considerable attention lately mainly because they can be useful for their potential application as solar-cell absorbers2-4, photocatalysts for solar water splitting5 and thermoelectric materials6-8. In particular, chalcogenide tin-sulphide complex compounds are very promising for optoelectronics, which is a consequence of high electron-phonon anharmonicity for such kind of materials9. One of these materials, Cu2ZnSnS4 has attracted great attention for photovoltaic devices because of its optimum direct band gap energy (∼1.5 eV), large absorption coefficient (∼104−6 cm−1, naturally abundant and environmentally friendly thin-film solar cell absorber10,11. These quaternaries fulfill the rules of adamantane compound formation, according to which the cation substitution is performed in such a way that the average number of valence electrons per atomic site and the ratio valence electrons to anions, which in diamond-like materials are four and eight, respectively, is preserved1.
Structural studies carried out on some members of this family indicate that they crystallize in sphalerite or wurtzite derivative structures. In sphalerite derivatives with tetragonal symmetry: in a Cu2FeSnS4-type structure (stannite, space space group
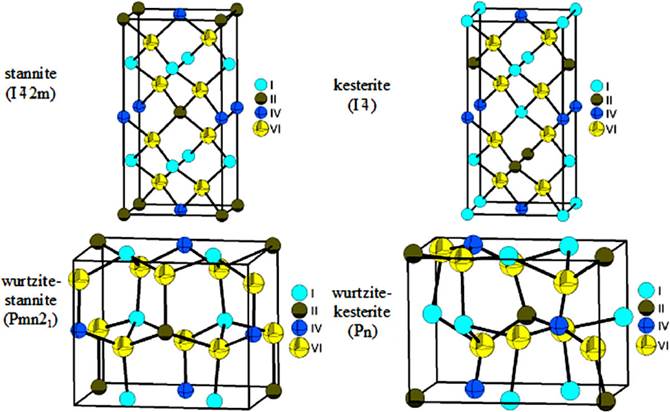
Figure 1 Unit cell diagram of the stannite, kesterite, wurtzite-stannite and wurtzite-kesterite structures showing the cation and anion distribution in the I2-II-IV-VI4 family of compounds.
Several recent studies on the structural characterization and physical properties, including transport properties, of these Cu2-II-IV-VI417-28 and Ag2-II-IV-VI429-35 quaternary semiconductor chalcogenides have been reported.
On the other hand, I2-II-IV-VI2 semiconducting compounds in which the II
cation is a paramagnetic ion, as Mn+2, Fe+2, Co+2
or Ni+2, known as semimagnetic materials, have also received considerable
attention because magneto-optical effects larger than those observed in II
1-x
Mn
x
-V
I2
alloys could be obtained in such quaternaries36. One of them, Ag2MnSnS4 could be
of interest because it exhibits antiferromagnetic behavior37. Concerning to its crystal structure, discrepancy
exists in the scarce information reported the lit-erature. According to Lamarche
et al.37,
this compound crystallizes with orthorhombic symmetry and unit parameters
Furthermore, Ag2MnSnS4 is one of the three sulfide minerals containing silver and tin found in nature. They are the hocartite, Ag2FeSnS4, found in Bolivia in 196739, the pirquitasite, Ag2ZnSnS4, found in Argentina in 198040, and the agmantinite, Ag2MnSnS4, found in Perú in 201441, whose name has been recently approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association41.
In view of the considerable importance of such semimagnetic compound, in this work we report the synthesis, thermal analysis and crystal structure characterization of the I2-II-IV-VI4 Ag2MnSnS4 semimagnetic compound.
2. Experimental
2.1. Synthesis
The sample was synthesized by the melt and anneals technique. Highly pure components (silver 99.98%, manganese 99.97%, tin 99.99%, and sulphur 99.99) of 1 g sample were sealed under vacuum (
2.2. Chemical Analysis
The stoichiometric relation of the sample was investigated by SEM technique, using a Hitachi S2500 microscope equipped with a Kedex EDX accessory. Three different regions of the ingot were scanned and the average atomic percentages are as follows: Ag (25.2%), Mn (11.1%), Sn (11.3%) and S (52.4%). The error in standardless analysis was around 5%. These values are in good agreement with the ideal composition 2:1:1:4.
2.3. Differential thermal analysis (DTA)
Phase transition temperatures were obtained in the temperature range between 20 to 1150°C, using a Perkin-Elmer DTA-7 with aluminum and gold used as reference materials. The charge was of powdered alloy of approximately 100 mg weight. Both heating and cooling runs were carried out on each sample. The average rates of these runs being approximately 10°C/min. The error in determining these temperatures is of about
2.4. X-ray powder diffraction (XRPD)
For the X-ray analysis, a small quantity of the sample, cut from the ingot, was ground
mechanically in an agate mortar and pestle, and sieved to a grain size of less
than 10 µm. The XRPD data was collected at 293(1) K, in
3. Results and Discussion
3.1. Differential thermal analysis
DTA runs were carried out on the sample as indicated above. The transition temperatures as well as the type of melting were obtained from the peaks on the DTA heating and cooling curves. Each transition temperature was determined from the base intercept of the tangent to the leading edge of the peak in the differential signal. The value of the melting point temperature, denoted as TM, obtained from the peaks on the DTA curve, is given in Fig. 2, which shows the thermogram for Ag2MnSnS4.
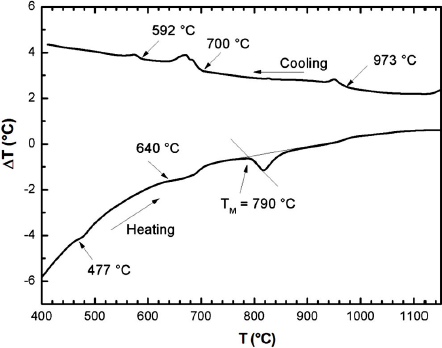
Figure 2 DTA thermogram for Ag2MnSnS4. The direction of heating run, or cooling run, is indicated by the corresponding arrow
Three transitions are observed in both heating and cooling runs. These appear at about 477, 640 and 790°C, and at about 973, 700 and 592°C, in these curves, respectively. It can also be seen, that the compound melts congruently at about 973°C. Hence, the effect observed at 973°C corresponds to the transition from (L +
3.2. X-ray powder diffraction analysis
Figure 3 shows the resulting X-ray powder diffractogram for the Ag2MnSnS4 compound. The X-ray powder pattern show a single phase. The 20 first peak positions were indexed using the program Dicvol0442, which gave a unique solution in an orthorhombic cell with parameters
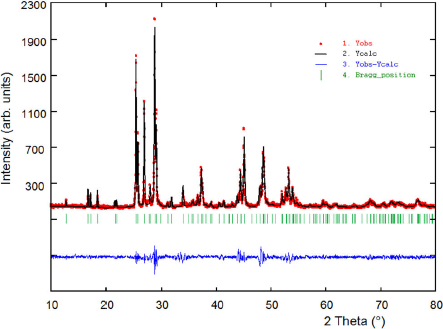
Figure 3 Final Rietveld plot showing the observed, calculated and difference pattern for the Ag2MnSnS4 compound. The Bragg reflections for both phases are indicated by vertical bars.
The Rietveld refinement43 was performed using the Fullprof program44. The indexed unit cell results were taken as starting parameters. The angular dependence of the peak full width at half maximum (FWHM) was described by the Cagliotti’s formula45. The parameterized Thompson-Cox-Hastings pseudo-Voigt profile function46 was used for the simulation of the peak shapes. The background of the XRD data was refined with a polynomial with six coefficients. The thermal motion of the atoms was described by one overall isotropic temperature factor. A total of 23 parameters of the Ag2MnSnS4 compound, including peak shape, scale factor, cell, atomic coordinates, isotropic displacement and full-width at half-maximum (FWHM) parameters, were refined. The final Rietveld refinement led to agreement factors of: Rp = 10.3%, Rwp = 11.8%, Rexp = 9.6%, and S = 1.2, for 4001 step intensities and 145 independent reflections.
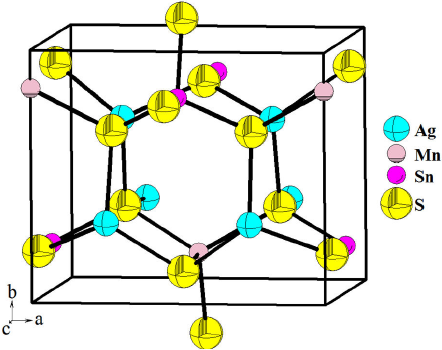
The results of the Rietveld refinement are summarized in Table I. Figure 3 shows the observed calculated and difference profile for the final cycle of the refinement. Atomic coordinates, occupancy factors and isotropic temperature factor are given in Table II. Bond distances and angles are given in Table III. Figure 4 shows the unit cell diagram for Ag2MnSnS4.
Table I Results of Rietveld refinement for Ag2MnSnS4.
| Molecular formula | Ag2MnSnS4 | Dcalc | 4.55 (g/cm3) |
| Molecular weight | 517.7 (g/mol) | Nº step intensities | 4001 |
| Crystal system | Orthorhombic | Nº independent reflections | 145 |
| Space group | Pmn21 (Nº 31) | Peak-shape profile | Pseudo-Voigt |
| Z | 2 | ||
| a | 8.1705(5) (Å | Rexp 9.6 % | |
| b | 6.9413(5) Å | Rp | 10.3 % |
| c | 6.6532(5) Å | Rwp 11.8 % | |
| V | 377.33(5) | S | 1.2 |
Table II Atomic coordinates, occupancy factors and isotropic temperature factor for Ag2MnSnS4.
| Atom | Ox. | Wyck. | x | y | z | foc | B (Å2) |
| Ag | +1 | 4b | 0.256(2) | 0.317(2) | 0 | 1 | 0.6(1) |
| Mn | +2 | 2a | 0 | 0.848(2) | 0.988(2) | 1 | 0.6(1) |
| Sn | +4 | 2a | 0 | 0.185(2) | 0.491(2) | 1 | 0.6(1) |
| S1 | -2 | 4b | 0.237(2) | 0.325(2) | 0.387(2) | 1 | 0.6(1) |
| S2 | -2 | 2a | 0 | 0.186(2) | 0.821(2) | 1 | 0.6(1) |
| S3 | -2 | 2a | 0 | 0.884(2) | 0.365(2) | 1 | 0.6(1) |
Table III Distance lengths (Å) and bond angles (º) for Ag2MnSnS4.
| Ag-S1 | 2.58(1) | Mn-S1iii | 2.55(2) | Sn-S1 | 2.27(2) |
| Ag-S1i | 2.60(2) | Mn-S1vi | 2.55(2) | Sn-S1vii | 2.27(2) |
| Ag-S2ii | 2.57(2) | Mn-S2iv | 2.60(2) | Sn-S2 | 2.20(2) |
| Ag-S3i i | 2.59(2) | Mn-S3v | 2.52(2) | Sn-S3viii | 2.25(2) |
| S1-Ag-S1i | 105.6(4) | S1-Ag-S2ii | 114.9(3) | S1-Ag-S3i | 113.8(3) |
| S2ii -Ag-S1i | 102.8(3) | S1i -Ag-S3i | 113.4(3) | S2ii -Ag-S3i | 105.9(3) |
| S1iii -Mn-S1vi | 114.7(5) | S1iii -Mn-S2iv | 108.2(3) | S2iv -Mn-S1vi | 108.2(3) |
| S3v -Mn-S1iii | 108.0(3) | S3v -Mn-S1vi | 108.0(3) | S3v -Mn-S2iv | 109.7(7) |
| S2-Sn-S1 | 107.6(3) | S2-Sn-S3viii | 112.0(8) | S1-Sn-S3viii | 106.5(4) |
| S1vii -Sn-S2 | 107.6(3) | S1vii -Sn-S1 | 116.7(6) | S1vii -Sn-S3viii | 106.5(4) |
Symmetry codes: (i) 0:5 - x, 1 - y, -0:5 + z; (ii) x, y, -1 + z; (iii) -0:5 + x, 1 - y, 0:5 + z; (iv) x, 1 + y, z; (v) x, y, 1 + z; (vi) 0:5 - x, 1 - y, 0:5 + z; (vii) -x, y, z; (viii) x, -1 + y, z.
Ag2MnSnS4 crystallize with orthorhombic symmetry, space group
The tetrahedrons containing the Sn atoms [mean S
The mean bond distance values for Ag-S, Mn-S and Sn-S, given in Table III, agree well with the distances observed in other adamantane compounds as Ag2CdSnS49, Ag2CdGeS429, Ag2CdSnS430, Ag2ZnSiS432, AgCd2GaS448, Ag2ZnGeS449, MnIn2S450, AgInS2 and AgIn5S851.
In addition, the Debye temperature
Conclusions
The quaternary chalcogenide compound Ag2MnSnS4 crystallizes in the wurtzite-stannite structure, space group











 nueva página del texto (beta)
nueva página del texto (beta)