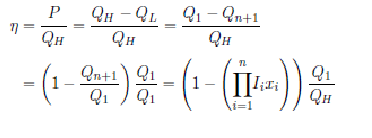1. Introduction
Recently in1, a methodology of optimization was applied to an irreversible Carnot-like power plant, where the characteristic parameters were: the allocation or effectiveness of the heat exchangers of this plant. Although this methodology is applicable to any Carnot-like power plant, a standard irreversible Carnot-like cycle was chosen because of its simplicity to account for the main irreversibilities that usually arise in real heat engines2: “finite rate heat transfer between the working fluid and the external heat sources, internal dissipation of the working fluid, and heat leak rate between reservoirs”. The above standard irreversible Carnot-like cycle has been studied at length for many objective functions, different transfer heat laws and several characteristic parameters (see2-24 for more details).
The maximum power and efficiency have been obtained in3-6. In general, these optimizations were performed with respect to only one characteristic parameter: internal isentropic temperature ratio (
On the other hand, n-stage combined Carnot cycle has been presented, by the law of heat conduction, in24-26 optimizing the specific power and efficiency for the characteristic parameters: isentropic temperature ratios , and effectiveness (other combined cycles can be found in27-36, coupled heat devices in37-38; see also the references therein included).
In this paper we extend the results of allocation or effectiveness for one cycle to two or more combined cycles of the same irreversible Carnot-like power plant using iteratively the Bellman’ Principle39 which has been successfully applied in Refs. 13 and 14. We found optimal recurrence relations remarkable for the irreversible power plant with n Carnot-like cycles for two constraints (design rules): constrained internal thermal conductance or fixed total area of the heat exchangers from hot and cold side.
This paper is organized as follows. In Sec. 2 we present the irreversible power plant model and the functional relation between power and efficiency which extends to the corollary presented in1 to the irreversible power plant. Section 3 presents the optimization of the allocation and effectiveness, by two design rules, of heat exchangers for the Carnot-like 𝑛-cycles of the power plant. Section 4 is devoted Conclusions.
2. Power plant with n Carnot-like cycles
The power plant with n Carnot-like cycles is shown in Fig. 1. Each cycle satisfies the conditions expressed in2,4: leak heat rate Q and finite heat transfer rates Qi and internal dissipations of the working fluid expressed by constants
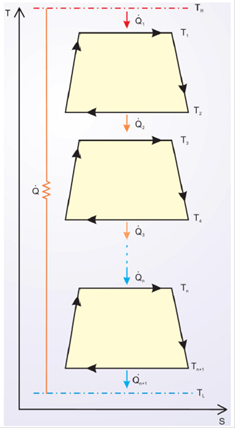
Figure 1 A power plant with n Carnot-like cycles, with heat leak rate, finite heat transfer rates, and internal dissipations of the working fluid in each cycle.
Following to1,25, the thermal efficiency of the power plant is given by (this functional form for only a cycle has appeared in Refs. 1, 2, and 17):
where
The Eq. (1) is obtained of the following way: according to the second Law of the Thermodynamics, for each
Thus,
Now, using the Eqs. (3) and (4), the efficiency is given by,
From the Eq. (5),
And from the Eq. (6), (1) is obtained.
The Eq. (1) extends to the Eq. (6) of1. Therefore, we can seek, as in1, invariant optimal relations, by following the spirit of Carnot’s work, for other characteristic parameters, which can be independent from the heat transfer law, of the irreversible power plant.
Also, the Eq. (1) say us that it is enough analyzes only for power and efficiency (for other operation regimes, e.g. algebraic combination of power and/or efficiency that have thermodynamic meaning and satisfy imposed power conditions: see Eq. (7) below and for more details see1). For instance, the functional expression of the efficiency, power and the heat transfer, without require of algebraic explicit expressions, have been used in2, for two constraints (design rules): constrained internal thermal conductance or fixed total area of the heat exchangers from hot and cold side. The isentropic temperature ratio do not has this condition1,2,3,13,24-26.
Now, if we take
“The power 𝑃 achieves a maximum value in 𝑧 𝑚𝑝 if and only if the efficiency 𝜂 achieves a maximum value in the value:
Thus, in the optimization of power plant, with respect to 𝑧 it is enough to find the maximum power by,
The optimization performed, with respect to will be: a property independent from the heat transfer law. This a remarkable conclusion of the Eq. (1) is that it can find the maximum for one and only one the power (see the criterion of2). For example the power, for power plant model, by
We show the above as follows: Let
The power and the efficiency satisfy the functional relationship given by the Eq. (1). Deriving (1) with respect to:
since, we can suppose that Q does not depend of the variable z.
Therefore,
where
In all the above calculations none transfer heat law has been used.
Therefore, it is enough to have one, and only one, algebraic expression of the power and
any heat transfer law. We choose for our convenience the power
and the conduction heat transfer Law since they are algebraically the simplest. In
the optimization of the power of the plant for two constraints (design rules):
constrained internal thermal conductance (
3. Universal optimal relations for the allocation and effectiveness of the heat exchangers of the power plant
The optimization of power and efficiency with respect to is well known for combined cycles13,24-26. Henceforth, x will be fixed and we will assume that the law of heat transfer rate can be any law, included also the heat leak rate. Essentially, we will treat the following two design rules: internal thermal conductance fixed; or areas fixed for heat exchangers; which will be applied alternatively.
The first design rule is that the internal conductance of the Carnot-like cycle is constrained to:
where Ai,Ai+1 are heat transfer areas on hot/cold sides of the cycle i(i = 1,2…n - 1).
Alternatively, the second design rule is that the total area is constrained by:
where
Indeed, the dimensionless power output (Eq. (2.5) for one cycle in3) for each cycle i is given by:
here
Next, applying the Bellman’ Principle39, in form iterative, we can obtain the optimal relations for the allocation of the heat exchangers of the power plant (Fig. 1). This principle will be applied as it is indicated in20: “to state that every part of an optimum path is optimal”.
3.1 Constrained internal thermal conductance
The 𝑛 thermal conductance can be written as:
where
Fixing the temperature
In parameterizing,
According to the Eq. (13), in optimizing
with respect to
since
Continuing of this way we arrive to the following optimal recurrence relation:
And
From the Eqs. (11) and (14) we have: the areas de transference of heat decreases from
and the equality is fulfilled if there is not internal dissipation in the n cycles.
3.2 Constrained areas of heat exchangers
For simplicity, we suppose
where
Then
where
From according to the Eq. (13):
the first optimization with respect to
Then,
Solving,
and, the results of1 are recovered.
Continuing of this way arrive us to the following optimal recurrence relation:
With
3.3 Some special cases
To illustrate the optimal relations (14 and 16) we can choose a power plant with 2 or 3 like-like-Carnot cycles:
a). Case constrained internal thermal conductance
In this case, from the Eq. (14), we obtain the conductance:
b). Constrained areas:
In this case, from the Eq. (16), we obtain the areas, for two cycles,
And for three cycles,
2 where
4. Conclusions
We have found and determined the optimal allocation of heat exchangers of a power plant with n Carnot-like cycles. We must note that the optimal recurrence relations (Eqs. (14 and 16)) found for this model are valid for any heat transfer law and have not been reported in the literature previously; specially the Eq. (16). Thus, following the spirit of Carnot’s work, we have found invariant optimal relations for power and efficiency maximum which are valid for any heat transfer law. Moreover, these relations can be satisfied for other operation regimes, e.g. algebraic combination of power and/or efficiency that have thermodynamic meaning and satisfy the conditions imposed to the power (Eq. (7)) which can be carried out as in1. Nevertheless, the optimal isentropic temperature ratios depend on the heat transfer law and the operation regime of the engine as is discussed in1,2,3,13,24-26.
Finally, the above equations can be extended to n Carnot-like cycles presented here including other heat transfer rate laws by the substitution of either of (14) or (16) in the objective function (algebraic combination of power and/or efficiency) and optimize only for the isentropic temperature ratio. However, the latter requires a comprehensive study of the implications for the power plant considered. We will study such implications as future work.











 text new page (beta)
text new page (beta)