PACS: 61.05.cp; 61.50.Nw, 61.66.Fn
1. Introduction
The compounds with ternary structures of the chalcopyrite family Cu-III-Se2 (III = Al, Ga, In) form an wide group of semiconductor materials with diverse optical and electrical properties 1. They crystallize with tetragonal symmetry in the space group I
The first crystal structure characterization of one I-II-III-VI3 semiconductor member, CuFeInSe3, indicated a degradation of symmetry from the chalcopyrite structure I
In this work, we report a detailed structural analysis of three new members of the Cu-II-III-Se3 family, CuZnAlSe3, CuZnGaSe3 and CuZnInSe3 which was performed using X-ray powder diffraction by means of the Rietveld method.
2. Experimental
Starting materials (Cu, Zn, Al, Ga, In and Se) with a nominal purity of (at least) 99.99 wt% in the stoichiometric ratio were mixed together in an evacuated and sealed quartz tube with inner walls previously carbonized. Polycrystalline ingots of about 1 g were prepared by the usual melting and annealing technique, lowering the temperature from 1500 to 850 K at a rate of 20 K/h, keeping this temperature for 30 days, and finally, cooling to room temperature at a rate of 10 K/h. Previous experience indicates that this procedure usually gives samples showing conditions corresponding to equilibrium near room temperature.
For the X-ray analysis, small quantities of the samples were ground mechanically in an agate mortar and pestle. The resulting fine powders, sieved to 106 µ, were mounted on a flat zero-background holder covered with a thin layer of petroleum jelly. The X-ray powder diffraction data were collected at 293(1) K, in θ/θ reflection mode using a Siemens D5005 diffractometer equipped with an X-ray tube (CuKα radiation: λ = 1.54056 Å; 40 kV, 30 mA) using a secondary beam graphite monochromator. A fixed aperture and divergence slit of 1 mm, a 1 mm monochromator slit, and a 0.1 mm detector slit were used. The specimens were scanned from 10°-100° 2θ, with a step size of 0.02° and counting time of 40 s. Quartz was used as an external standard.
3. Results and discussion
The X-ray diffractograms of three alloys CuZn-III-Se3 (III= Al, Ga, In) showed single phases. The powder patterns were indexed using the program Dicvol04 22, and tetragonal cells with similar magnitudes to the parent chalcopyrite structures, CuAlSe223, CuGaSe224, CuInSe225 were founds. Systematic absences are consistent with a P Bravais lattice. A detailed pattern examination taking in account the sample composition, cell parameters and lattice-type, suggested that all compounds are isostructural with previously reported CuFeInSe38; the first structural characterization of a I-II-III-VI3 semiconductor member, which crystallizes in the space group P
The Rietveld refinements 26 of the structures were carried out using the Fullprof program 27. The atomic coordinates of CuFeInSe38 were used as starting model for each refinement. The angular dependence of the peak full width at half maximum (FWHM) was described by the Caglioti’s formula 28. Peak shapes were described by the parameterized Thompson-Cox-Hastings pseudo-Voigt profile function 29. The background variation was described by a polynomial with six coefficients. The thermal motion of the atoms was described by one overall isotropic temperature factor. The results of the Rietveld refinement for the three alloys are summarizes in Table I. Figure 1 shows the observed, calculated and difference profile for the final cycle of Rietveld refinements. Atomic coordinates, isotropic temperature factor, bond distances and angles for each compound are shown in Tables II, III and IV.
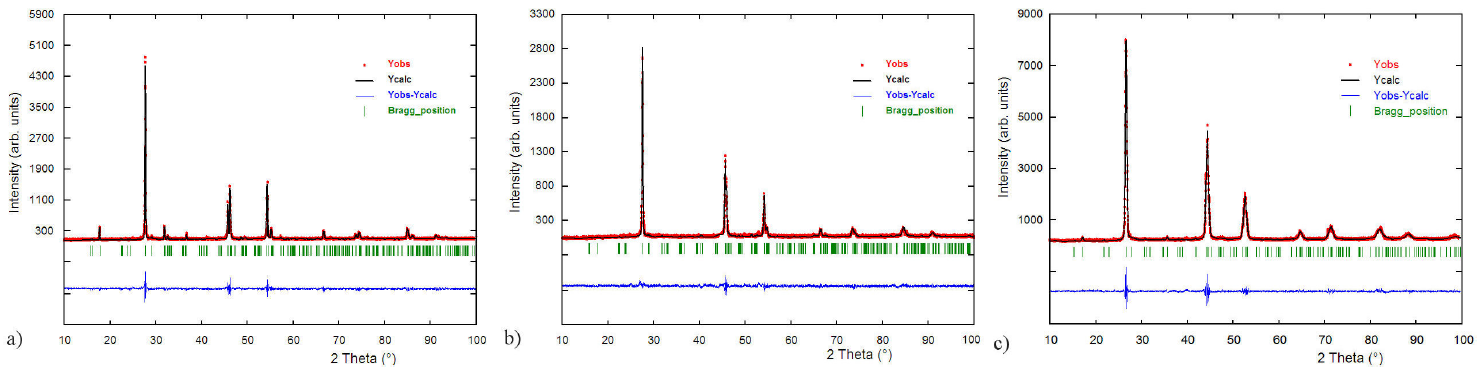
FIGURE 1 Rietveld final plots of a) CuZnAlSe3, b) CuZnGaSe3 and c) CuZnInSe3. The lower trace is the difference curve between observed and calculated patterns. The Bragg reflections are indicated by vertical bars.
TABLE II Atomic coordinates, isotropic temperature factors, bond distances (Å) and angles (◦) for CuZnAlSe3, derived from the Rietveld refinement.
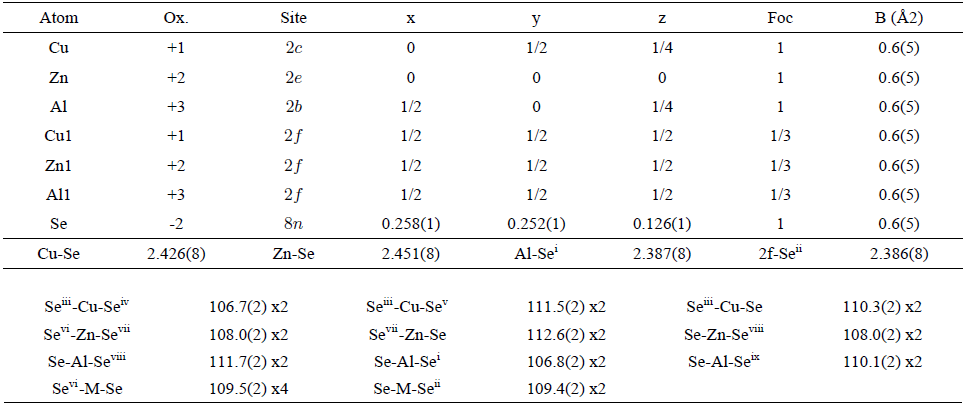
Symmetry codes: (i) 1-x, y, 0.5-z; (ii) 1-x, 1-y, z; (iii) -x, 1-y, z; (iv) -x, y, 0.5-z; (v) x, 1-y, 0.5-z; (vi) -y, x, -z; (vii) -x, -y, z; (viii) y, -x, -z; (ix) 1-x, -y, z.
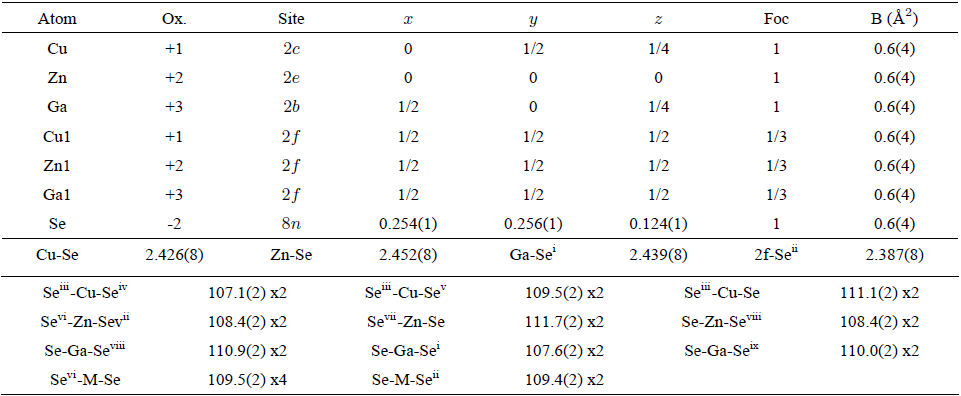
TABLE III Atomic coordinates, isotropic temperature factors, bond distances (Å) and angles (◦) for CuZnGaSe3, derived from the Rietveld refinement.
Symmetry codes: (i) 1-x, y, 0.5-z; (ii) 1-x, 1-y, z; (iii) -x, 1-y, z; (iv) -x, y, 0.5-z; (v) x, 1-y, 0.5-z; (vi) -y, x, -z; (vii) -x, -y, z; (viii) y, -x, -z; (ix) 1-x, -y, z.
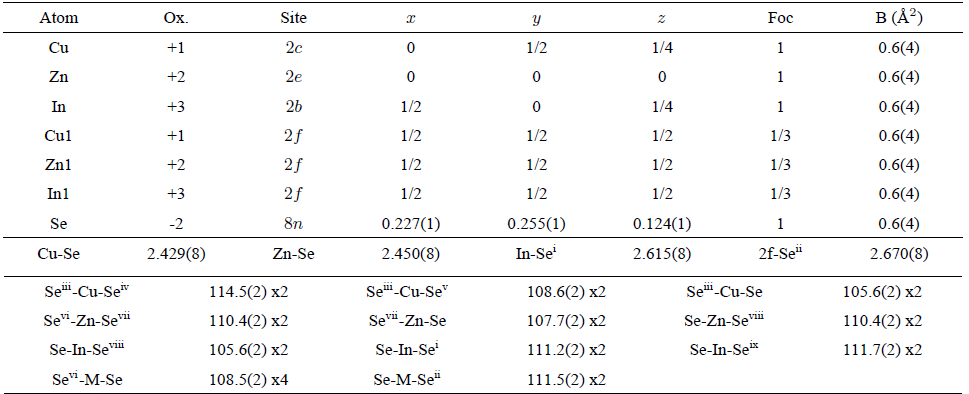
TABLE IV Atomic coordinates, isotropic temperature factors, bond distances (Å) and angles (◦) for CuZnInSe3, derived from the Rietveld refinement
Symmetry codes: (i) 1-x, y, 0.5-z; (ii) 1-x, 1-y, z; (iii) -x, 1-y, z; (iv) -x, y, 0.5-z; (v) x, 1-y, 0.5-z; (vi) -y, x, -z; (vii) -x, -y, z; (viii) y, -x, -z; (ix) 1-x, -y, z.
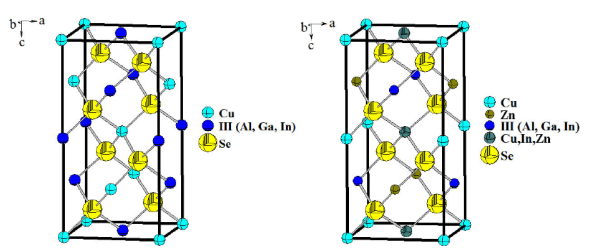
CuZnAlSe3, CuZnGaSe3 and CuZnInSe3 are normal adamantane-structure compounds 2 related to the CuAlSe2, CuGaSe2 and CuInSe2 chalcopyrite parent structures, where the Zn cation are “diluted” leaving the cell volume almost unchanged. Figure 2 show the unit cell diagram for the Cu-Zn-III-Se3 compounds compared to the chalcopyrite Cu-III-Se2 parents, and Table V show a comparison between the unit cell parameters and the bond distances for the three phases of both families of compounds.
TABLE V Comparative table of unit cell parameters and bond distances for the Cu-III-Se2 chalcopyrite compounds and the related Cu-Zn- III-Se3 alloys (III= Al Ga, In). ([ * ] = this work).

In CuZnAlSe3, the tetrahedra containing the Cu atoms [mean Se…Se distance 3.97(1) Å] are slightly smaller than those containing the M (Cu1 or Zn1 or Al1) atoms [means Se…Se distance 4.20(1) Å], Al atoms [means Se…Se distance 3.97(1) Å], and Zn atoms [mean Se…Se distance 4.00(1) Å], respectively. In CuZnGaSe3, the tetrahedra around the cations, in mean Se…Se distance, are: M (Cu1, Zn1, Ga1) 3.90(1) Å, Cu 3.96(1) Å, Ga 3.90(1) Å, and Zn 4.00(1) Å, respectively. In CuZnInSe3, the tetrahedra around the cations, in mean Se…Se distance, are: M (Cu1, Zn1, In1) 3.94(1) Å, Cu 3.97(1) Å, In 3.91(1) Å, and Zn 4.10(1) Å, respectively.
The Cu-Se, Zn-Se and III (Al,Ga,In)-Se bond distances in all compounds (Table II, III and IV) are in good agreement with those observed in the parent chalcopyrite structures (Table V) and other quaternary adamantane structure compounds such as CuFe(Al,Ga,In)Se38,17, CuFe2 (Al,Ga,In)Se414,19, Cu2FeSnSe430, Cu2ZnGeSe431 and Cu2ZnSnSe432.











 text new page (beta)
text new page (beta)