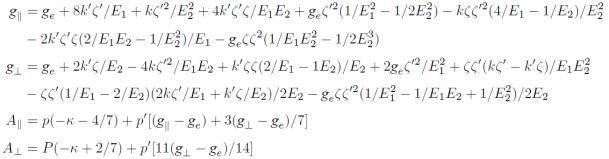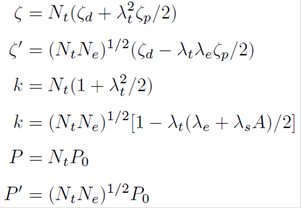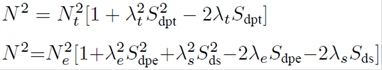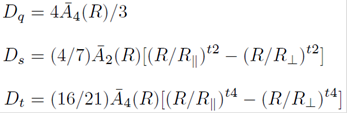PACS: 75.10.Dg; 71.70.ch; 76.30.Fc
1. Introduction
Mixed-alkali effect in glasses has received much attention with respect to its technological and theoretical interests in describing the physics and chemistry of glasses which are of the potential applications in the development of new tunable solid-state lasers, solar-energy converters, and fiber-optic communication devices 1-3. It is well known that the transition metal ions are conveniently applied as probes to provide useful information about the local structures of glasses, due to their sensitive response to surrounding actions. For example, Cu 2+ ion (3d 9 ) with one 3d hole is normally treated as a model system of specific significance, 4 containing a single ground state and a single excited state under ideal octahedral crystal field and is frequently applied as probes in the EPR experiments 5-9 Giridhar et al., performed the EPR experiments and optical absorption studies for Cu 2+ ion in mixed alkali cadmium phosphate glasses, 8 and the EPR parameters (the anisotropic g factors gǁ and g┴ and the hyperfine structure constants A║ and A┴ ) were also measured for the center 8. However, until now, the local structure of the impurity center in the glasses has not been obtained, except that the observed EPR results were tentatively assigned to the tetragonally elongated Cu2+ center. In addition, the optical spectra were not interpreted. Since the microscopic information about the local structure and the EPR behaviors (particularly the g factors) for Cu2+ in mixed alkali cadmium phosphate glasses would be helpful to understand the nature and symmetry of the glasses, theoretical studies on the above EPR results are of fundamental and practical significance. In this work, the EPR parameters of Cu2+ center in mixed alkali cadmium phosphate (10Li2O-10Na2O-20CdO-59.5P2O5, LiNaCdP hereafter) glass are theoretically investigated by using the high order perturbation formulas of these parameters for a 3d9 (Cu2+) ion under tetragonally elongated octahedra. In these formulas, the ligand orbital contributions are considered and the energy separations are correlated with the local structure. Thus, the optical spectra can be interpreted and hence information of the local structure for the impurity ion Cu2+ obtained on the basis of the EPR analysis. The results are discussed.
2. Calculation
When the impurity Cu2+ ions are doped into mixed alkali cadmium phosphate glasses, they prefer to occupy the Cd2+ site due to the same charge and form the octahedral [CuO6]10- clusters 8. For a Cu2+ ion under octahedral symmetry, there are only two energy levels, i.e., one lower orbital doublet 2Eg $^2 and another higher orbital triplet 2T2g. Then, the two-fold orbital degeneracy of 2Eg ground state can be canceled by the Jahn-Teller effect via vibrational interactions, which always correlates to the removal of the degeneracy of energy levels and results in lower symmetry and energy 10-12 The doped Cu2+ ions are found to occur in tetragonally elongated along certain direction (C4 axis of the systems) and lead to the local tetragonal point symmetry 8. For Cu 2+ (3d9) ion in tetragonally elongated octahedra, the lower 2Eg level would be split into 2B2g and 2A1g, with the former being lowest 4. Meanwhile, the upper 2T2g energy level would be separated into 2B2g and 2Eg. According to the high order perturbation theory and considering the contributions from the ligand orbital and spin-orbit coupling interactions due to covalency effect between the central ion and ligand ions, the high-order perturbation formulas of the EPR parameters for a 3d9 ion in tetragonally elongated octahedra can be established from the cluster approach 13,14,15
Here, ge ≈2.0023 is the spin-only value, P and P’ are the dipolar hyperfine constant related to the interaction within t2g states and the interaction between t2g and eg states.K is the core polarization constant. E1 and E2 are the energy separations between the excited 2B2g , 2Eg and the ground 2B1g states, they can be expressed in terms of the cubic field parameter Dq and the tetragonal field parameters Ds and Dt . Thus, we have:
Based on the cluster approach, the spin-orbit coupling coefficients ζ and ζ′ and the orbital reduction factors k’ and k and the dipolar hyperfine structure parameters P and P’ in the formulas of EPR parameters can be expressed as: 13,16
In above formulas, ζ d and ζ p are, respectively the spin-orbit coupling coefficients of the free 3d9 and ligand ions. P0 (≈388X10-4 cm-117) is the dipolar hyperfine structure parameter of free Cu2+ ion. A denotes the integral R<ns|∂/∂y|npy >, where R is the impurity-ligand distance of the present system. Ny and λy (or λs ) are the normalization factors and the orbital mixing coefficients for the cubic irreducible representations γ(=eg or t2g ) They can be determined from the approximate relationships: 13,16
Here N is the average covalency factor, characteristic of the covalency effect of the central ion in crystals. Sdpy (and Sds ) are the group overlap integrals. In general, the orbital mixing coefficients increase with increasing the group overlap integrals, and one can approximately adopt proportionality relationship λe/Sdpe≈λs/Ss between the orbital mixing coefficients and the related group overlap integrals within the same irreducible representation eg.
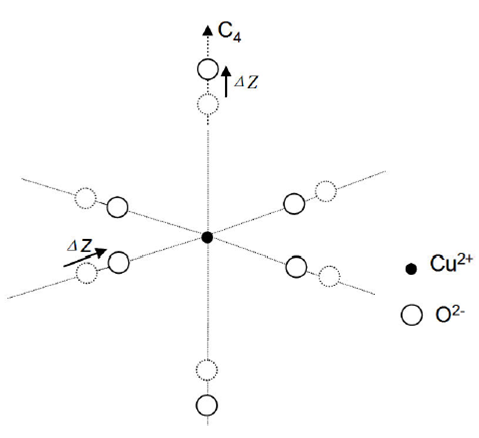
As mentioned before, the Jahn-Teller Cu2+ ion can suffer the Jahn-Teller effect via stretching the two Cu2+O2- bonds along the C4 axial direction and then lead to the local tetragonal point symmetry. So, the parallel and perpendicular Cu2+-O2 bond lengths can be given in terms of the reference distance R and the elongation ΔZ along the C4 axis as R║=R+∆Z and R ┴=R-∆Z (see Fig. 1). Thus, the cubic and tetragonal field parameters (Dq, Ds and Dt ) can be determined from the superposition model18 and the geometrical relationship of the studied impurity Cu2+ center:13-15
Here t2 (≈3) and t4 (≈5) are the power-law exponents, Ᾱ2 (R) and Ᾱ4 (R) are the intrinsic parameters with the reference bonding length R, here we estimate reasonably the reference distance R(≈2.04Å) for the Cu2+ center to be close to the sum of ionic radii of Cu2+ and O 2- ions 19, due to the electrostatic attraction between two ions 20,21,22. For 3d n ions in octahedra, the relationship Ᾱ2 (R)≈Ᾱ4 (R) is proved to be valid and is reasonably applied here 14,23.
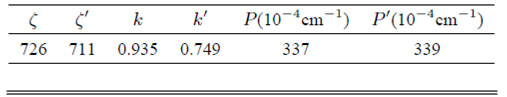
From the distance R and the Slater-type SCF functions,24,25 the group overlap integrals Sdpt , Sdpe , and Sds and the integral A are calculated and shown in Table I. Then, the molecular orbital coefficients Ny and λy can be obtained from Eq. (4), Eq. (5) and the covalency factor N(≈0.86) which can be determined from the relationship: N2≈1-h(L)k(M) (here the parameter h(L)(≈1) is the characteristic of the ligand O 2- and k(M) (≈0.26) is the characteristic of the central metal ion Cu 2+ ) 26,27. The calculated results are also shown in Table I. The spin-orbit coupling coefficients ζ and ζ′ and the orbital reduction factors k and k’ can be determined from Eq. (3) and the corresponding free-ion values ζ d (Cu 2+ )≈829 cm -1 , 11 ζ p (O 2- )≈151°cm-1 , 16 they are collected in Table II.
TABLE I The group overlap (and A) integrals, the molecular orbital coefficients Nγ and λγ and λs for Cu2+ center in LiNaCdP glass.

TABLE II Spin-orbit coupling coefficients (in cm−1), the orbital reduction factors and the dipolar hyperfine structure parameters for Cu2+ center in LiNaCdP glass.
In the formulas of the hyperfine structure constants, the core polarization constant k can be determined from the relationship: k≈-2X/(3<r-3 >), 16,17,26 here χ is the characteristic of the density of unpaired spins at the nucleus of the central ion and<r-3 > is the expectation value of inverse cube of the 3d radial wave function. From the data <r-3 >≈8.252 a.u. 4 and X≈-3.40 a.u. 17 for Cu2+ in some oxides, one can estimate k≈0.275 for the studied system which is close to the expectation value (≈0.3) for the 3d n ions incrystals 11,12,17 and can be regarded as reasonable.
Thus, there are only two unknown parameters, the tetragonal elongation ∆Z and the intrinsic parameter Ᾱ4(R) in the formulas of the EPR parameters. Substituting the related values into Eq. (1) and fitting the theoretical results to the experimental data, we have:
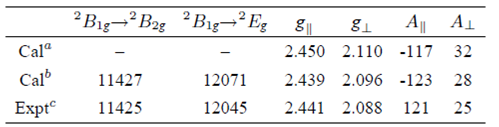
Accordingly, the parallel and perpendicular Cu-O distance R║ and R ┴ in the [CuO6]10- cluster are 2.051 Å and 2.029 Å respectively. The corresponding theoretical results of the optical absorption bands and the EPR parameters are shown in Table III. For comparison, the theoretical results based on the elongation ∆Z in Eq. (7) and neglecting the ligand contributions (i.e., ζ=ζ=N ζ d and =k’=N) are also collected in Table III.
TABLE III The optical spectral band positions (in cm−1) and EPR parameters (A constants are in units of 10−4 cm−1) for Cu2+ ions in LiNaCdP glass.
a Calculation based on the elongation ∆Z and neglecting the ligand contributions.
b Calculation based on the elongation ∆Z and inclusion of the ligand contributions.
c Reference 8
3. Discussion
From Table III, one can find that the calculated results (i.e., optical absorption spectra and EPR parameters) for Cu 2+ in LiNaCdP glass based on the elongation ∆Z and the intrinsic parameters Ᾱ4(R) in Eq. (7) show good agreement with the experimental data. Thus, the optical and EPR spectra for the impurity Cu 2+ in the studied glass are satisfactorily and uniformly interpreted.
The results R║≈2.051 Å and R┴≈2.029 Å indicate that the studied [CuO6]10- cluster in LiNaCdP glass shows a tetragonal elongation distortion along the C4 axis, which is consistent with the ground state 2B1g based on the observed EPR results (g║>g┴>ge and |A║|>|A┴). This point is also supported by many experimental and theoretical EPR studies of Cu2+ in various oxide glasses and crystals 4-6,17,28,29. Thus, Cu2+ tends to exhibit tetragonal elongation distortion due to the Jahn-Teller effect under octahedral environments. In addition, the small value of ∆Z is also consistent with the slight energy difference ∆E (≈E2-E1≈620 cm-1) between the excited 2Eg and 2B2g states of the studied system based on Eq. (2) and Eq. (6). So, the tetragonal elongation ∆Z (≈0.011°Ȃ) obtained in this work can be regarded as reasonable. The calculations show that the present theoretical model is effective in the explanations of optical spectra, EPR parameters and local distortion structure for d9 ions in glasses.
The small covalency factor N(≈0.86<1) and moderate orbital admixture coefficients (λt≈0.407, λe≈0.326 and λs≈0.264) obtained here reveal moderate admixture between the metal and ligand orbitals of the studied Cu2+ center in LiNaCdP glass. This point is consistent with the theoretical calculated results (see Cal a and Cal b ) in Table III. The above results show that the contributions from the ligand orbitals and spin-orbit coupling interaction should be considered in the explanation of the EPR parameters for Cu 2+ ions in glasses due to moderate (or significant) covalency effect of the studied system,7 although the ligand spin-orbit coupling coefficient( ζ p ≈151 cm -1 ) is smaller than that of the central Cu 2+ ion ( ζ d ≈829 cm -1 )16. In fact, neglecting the ligand contributions can yield larger spin-orbit coupling coefficients and the orbital reduction factors and hence lead to larger g factors (see Eq. (1)). In addition, the agreements between theory and experiment for the hyperfine structure constants based on neglection of the ligand contributions are not as good as those including these contributions. Therefore, the formulas of the EPR parameters containing the ligand orbital and spin-orbit coupling contributions seem to be more applicable than the simple ones in the absence of these contributions for the investigations on the EPR parameters of impurity ions in covalent systems.
From Table III, one can also see that the absolute values of the hyperfine structure constants A ║ and A ┴ are in good agreement with the experimental data and the sign for the calculated A ║ is negative, but the observed value given in Ref. 8 is positive. It should be pointed out that the signs of hyperfine structure constants for dn (or ƒ n ) ions in crystals cannot be determined solely from EPR experiments. Therefore, many experiments give them as absolute ones 4,17,28 The signs suggested here are the same as those for Cu 2+ doped in many glasses and crystals 17,28-30 and can be regarded as reasonable.
4. Conclusions
The EPR parameters and the local structure of the tetragonal Cu 2+ center in mixed alkali cadmium phosphate glasses are theoretically investigated from the high-order perturbation formulas for a 3d 9 ion in a tetragonally elongated octahedron. Based on the studies, the oxygen octahedral around Cu 2+ ions are found to suffer the tetragonal distortion ∆R(=R║-R┴ ) of about 0.022 Å due to the Jahn-Teller effect. The d-d transition optical spectra of the Cu 2+ center are theoretically interpreted. The signs of A ║ and A ┴ are suggested.











 nueva página del texto (beta)
nueva página del texto (beta)