INTRODUCTION
Human immunodeficiency virus-1 (HIV-1) infection remains a global public health problem, particularly in low- and middle-income countries. With no vaccine or definitive cure, HIV infection must be controlled using a combination of antiretroviral therapy (ART) to limit the ability of the virus to replicate and delay clinical progression. However, HIV drug resistance (HIVDR) emerging in response to ART selective pressure is one of the biggest threats limiting its success.
For over three decades, ART has been based on the use of a combination of antiretroviral drugs (ARVs) that inhibit two viral enzymes encoded in the HIV-1 pol gene: protease (PR) and reverse transcriptase (RT). Despite their high power and effectiveness in reducing viral replication, long-term exposure, and incomplete adherence, in many cases, it can lead to the selection and accumulation of resistance-associated mutations (RAMs) in the HIV-1 genome1,2. Monitoring the type and frequency of RAMs in a population is important to inform ART usage and effectiveness and to investigate the evolution in the HIV-1 pol genomic segment under the selective pressure of ARVs3,4. In the heterosexual and pediatric population from Argentina, subtype B strains co-circulate with several BF recombinants with specific characteristics and mosaic structures that have been thoroughly studied by our group and by others over the last years5,6.
To study the nature and extent of HIVDR in children and adolescents in Argentina, we conducted a longitudinal analysis of RAMs and the context of B or BF HIV-1 subtypes in a total of 374 cases showing virologic failure to ART between 2006 and 2021.
METHODS
Study group
The study included 374 HIV-1 vertically infected children and adolescents who received treatment and follow-up at "Hospital de Pediatría JP Garrahan" (Buenos Aires, Argentina) between 2006 and 2021.
Virological parameters and HIV-1 sequence data were organized and stored using a bioinformatics tool called SISGEN-HIV7, which was developed for data management of the HIV cohort at the Garrahan Hospital. The project was approved by the Research Project Review Committee and the Garrahan Hospital Ethics Committee (Protocol Nr. 856).
HIV-1 pol genotyping and identification of RAMs
HIV-1 plasma viral load (pVL) was measured on plasma samples using a commercial quantitative test (COBAS® TaqMan® HIV-1 Test, version 2.0; Roche), with a limit of detection of 34 HIV-1 RNA copies/mL (Log10= 1.53). Samples with pVL over 500 copies/mL were processed for RNA extraction, subsequent amplification, and direct sequencing of a polPR-RT gene fragment spanning codons 1-99 of PR and 1-220 of RT with a clinically validated in-house modified protocol of a nested polymerase chain reaction previously described by our group8. HIV-1 polPR-RT sequences were analyzed to determine the presence of RAMs to protease inhibitors (PIs), nucleoside reverse transcriptase inhibitors (NRTIs), and non-NRTIs (NNRTIs) using the Stanford HIVDR database and the 2022 update of the IAS-USA9. The level of resistance to each ARV was predicted using the Stanford HIVdb algorithm (https://hivdb.stanford.edu/). For this study, we computed only one HIV-1 genotype per patient, which was the most recent one available.
RESULTS
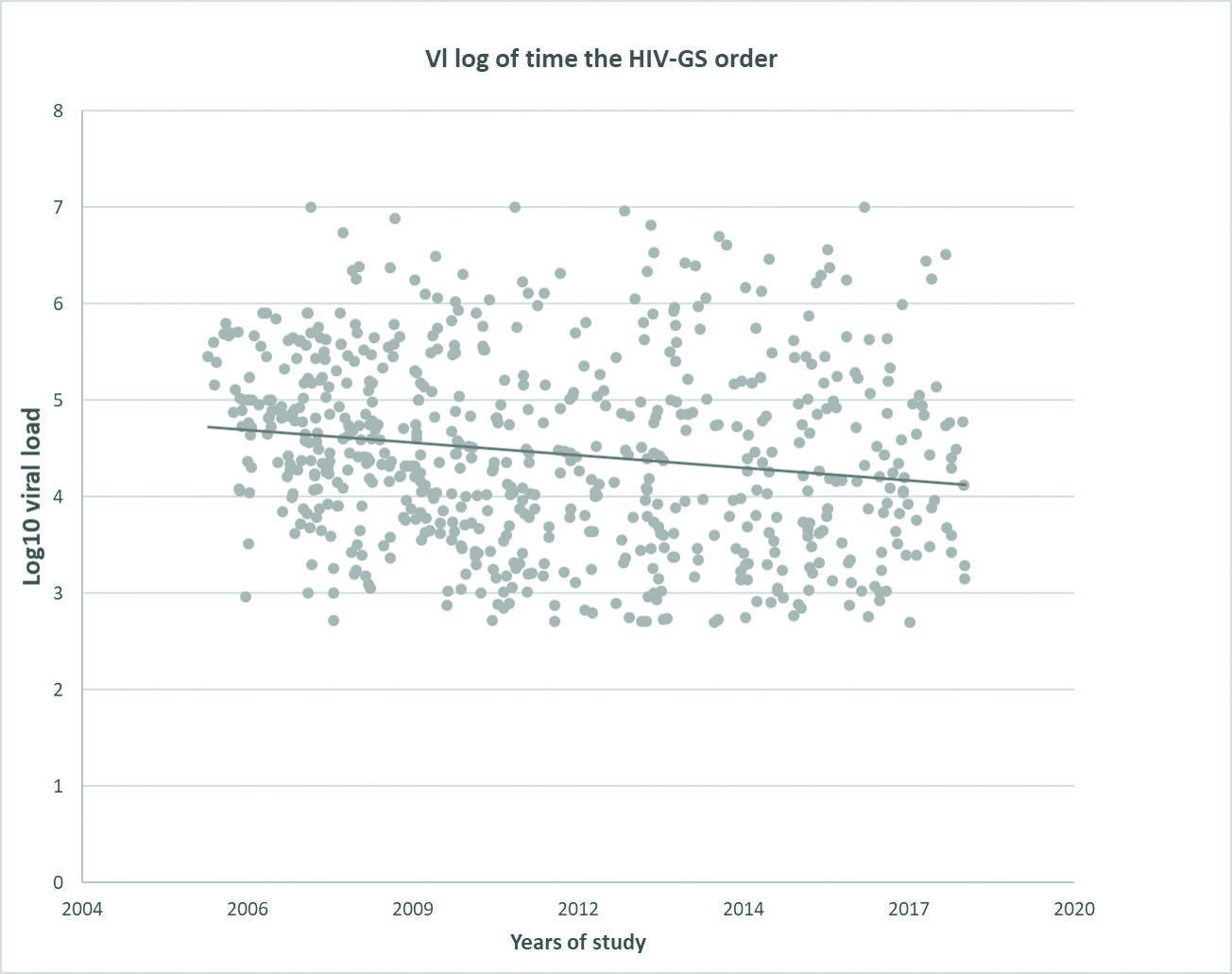
Between 2006 and 2021, 630 HIV genotypic resistance studies (HIV-GS) were performed to inform HIV-1 DR and guide changes in ART. HIV-1 pVL of the samples ranged between undetectable and above seven logs (Fig. 1), with a tendency toward lower pVL in samples remitted for HIV-GS during the most recent years. The sensitivity of amplification of our in-house HIV-GS is 100% for samples with pVL above 5.000 RNA copies/mL, 50% for samples with pVL between 1.000 and 5.000 RNA copies/mL, and 15% for samples with pVL below 1.000 RNA copies/mL.
Thus, the total number of patients who had samples with successful amplification of the HIV-1 polPR-RT gene fragment was 374 (59%). In most cases, patients had been exposed to NRTIs + NNRTIs (78%), NRTIs + PIs (89%), or all three ARV classes (85%) during their ART treatment. Patients in the study had an average age of 11 years (IQR = 1-22 years) and had been on ART for an average of 8.5 years (IQR = 0.3-17 years).
The level of acquired HIV-1 DR was analyzed for each of three classes of ARVs impacting PR and RT:PIs, NRTIs, and NNRTIs. Resistance to lamivudine (3TC) and emtricitabine (FTC) was considered independent since their resistance profile is mainly influenced by the RT M184V/I mutation and has limited cross-resistance with other thymidine analogs. Resistance to 3TC and FTC – as per the identification of M184V/I in sequences – was higher than 50% throughout the study period.
The dynamics of the percentage of resistance over time were evaluated using linear Poisson regression analysis (Fig. 2, Supplementary Material 1). Despite the persistently high proportion of cases with HIVDR to any ARV class throughout the study period (50%), we observed a decreasing trend in overall HIVDR (R2 = 0.24, p = 0.0474) (Fig. 2A). The high frequency of mutations suggests a direct link between virological failure and reduced susceptibility of HIV-1 to ART components. The frequency of resistance mutations was different for each of the classes of ARVs. A significant decrease in HIVDR prevalence was observed for both PIs and NRTIs. As shown in Fig. 2B, the prevalence of HIVDR to PIs decreased by more than 50% (from 67% to 33%) (R2 = 0.52, p = 0.0012). Similarly, the prevalence of HIVDR to NRTIs decreased by almost 20% (from 85% to 66%) (R2 = 0.30, p = 0.0225) (Fig. 2C) In contrast, HIVDR to NNRTIs remained moderate to high, ranging between 33% and 76% (Fig. 2D), with no signs of decline over time (p > 0.05).

Figure 2. Linear regression of the percentage of sequences with resistance mutations between the years 2006 and 2021. The X-axis represents the years studied. The Y-axis represents the percentage of sequences with resistance to A: at least one antiretroviral class, B: to protease inhibitors, C: to nucleoside reverse transcriptase inhibitors (excluding the M184V mutation), D: to non-nucleoside reverse transcriptase inhibitors.
The phylogenetic and bootscanning analyses of 357 out of the 374 HIV-1 pol fragments showed that 304 (85%) were BF recombinants, whereas the remaining 53 (15%) were pure subtype B without evidence of recombination. More than 90% of the BF recombinants were mosaic structures with a subtype B backbone, and subtype F genomic segments between PR codons 36-53 and RT codons 87-131 (Fig. 3A). Of the 48 RAMs identified in the dataset (Fig. 3B), 19 were associated with PIs, 18 with NRTIs, and 11 with NNRTIs. Among PI-associated mutations, D30N, M46I/L, I54V, and L90M predominated with frequencies ranging from 16% to 26%. All of them were found more frequently in BF recombinants, although statistically significant differences were observed only for I54V mutation (B: 9% vs. BF: 22%, p = 0.0037).

Figure 3. Analysis of mutations in subtype B and BF recombinants. A: percentage of sequences BF recombinants with B or F genomic segments along the pol PR-RT gene by boot scanning of Simplot. The X-axis shows the region 1-99 of PR and 1-200 of RT. B: frequencies of mutations between subtype B and BF recombinants. The dashed line divides resistance-associated mutations (RAMs) belonging to PR (left) and RT (right). The filled circle indicates the RAMs belonging to the thymidine analog mutation 1 profile. Stars indicate RAMs with significant differences between subtype B and BF recombinants.
For NRTIs, M184V was the most prevalent mutation (61%), with no differences between subtypes. Other common NRTI mutations were M41L, K70R, L210W, and T215Y. These belong to a profile of multi-DR to thymidine analogs, also known as thymidine analog mutation 1 (TAM 1), occurring more frequently in BF recombinants (B: 61% vs. BF: 80%, p = 0.0106).
For NNRTIs, mutations K103N/S and Y181C/I were highly frequent in both subtypes. However, a preference for BF recombinants was observed for K103S (B: 12% vs. BF: 4%, p = 0.0482).
DISCUSSION
The main cause of ART failure in HIV-1-infected patients is HIVDR. In this study, we describe the type and extent of acquired HIVDR in a population of HIV-1-infected children and adolescents receiving treatment and care at a referral care center in Argentina between 2006 and 2021. Our results show a progressive decline in HIVDR over time for PI- and NRTI-associated mutations, which can be explained in part by stricter criteria for defining virologic failures during treatment of HIV-1 infection that reduces the accumulation of drug-RAMs over time10. Few differences were observed between RAMs among B and BF recombinants, with a low overall impact on the level of DR.
Several studies have shown that children have a higher risk of selecting resistant variants during ART than adults11,12, with a high prevalence of RAMs even during their first ART regime13. This is mainly due to the difficulty in achieving optimal treatment adherence. The lack of pediatric formulations of ARVs, their poor palatability, and the absence of fixed-dose combinations for children are some of the factors that lead to poor adherence and consequently contribute to the development of viral resistance in this population. In our study, accumulation of RAMs was more frequent in the initial stage of the study, in association with higher levels of HIV-1 replication in plasma and less strict criteria for defining virologic failure.
Current HIV-1 treatment guidelines indicate the need to study HIVDR in patients who, after achieving complete virologic suppression on ART, demonstrate a confirmed viral load increment above the limit of detection of the assay. Our study confirms a lower probability of selecting resistant HIV-1 variants in this scenario. This is because viral replication is limited and controlled more rapidly with more powerful ARV options.
The frequency of resistance to different classes of ARVs in our population can be explained at least in part by the changes in guidelines and recommendations for ART in children over the past 15 years. In the 2000s, the PIs were recommended both for the ARV naive and ARV-experienced populations. Nelfinavir, lopinavir, ritonavir, and saquinavir were commonly used during this period, accounting for the selection of mutations at codons 30, 90, 46, and 84 in a high number of cases. By the late 2000s, most PIs were replaced by less toxic and more powerful drugs such as the first integrase inhibitors and new second-generation NNRTIs13. The use of PIs is currently limited to lopinavir, darunavir, and atazanavir (with low-dose ritonavir as a booster), which are recommended only as alternative ART regimes. Hence, the gradual replacement of PIs as part of ART in children provides a plausible explanation for the significant reduction in the number of PI-associated RAMs. HIVDR to NNRTIs showed a moderate decrease throughout the study period, but this was not statistically significant. K103N was the NNRTI-associated mutation with the highest frequency, in agreement with a selection of this mutation in other populations exposed to nevirapine and efavirenz – the first-generation NNRTIs – around the world14. In our population of vertically infected children, exposure to these ARVs could occur not only through ART but also by nevirapine as part of extended ARV prophylaxis in the mother-child pair to prevent the transmission of HIV-1. Mother-to-child transmission of NNRTI resistance has been documented at levels as high as 22% in our population15. This high frequency of transmitted HIVDR added to the long persistence of K103N even after treatment discontinuation16 is probably the main driver of the persistence of mutations associated with high levels of HIVDR to first-line NNRTIs in our population. It is important to highlight that, these viruses retain full susceptibility to second-generation NNRTIs such as etravirine or doravirine.
Classic ART involves the use of two NRTIs and an ARV of a different class. In recent years, this has been superseded by other regimens that do not include NRTIs, likely contributing to the observed decrease in NRTIs resistance. On the other hand, the persistence of the M184V mutation at high frequency can be explained by the widespread use of 3TC as part of classic ART regimens. While maintaining this ARV as part of ART was once considered beneficial for reducing viral fitness by selecting for the M184V mutation, this concept is still under debate17,18.
The consensus mutation lists, bioinformatics tools, and most of the current knowledge of HIV-1 used in this study are based on subtype B. This represents a challenge for the correct management of the remaining subtypes of HIV-1, which corresponds to 90% of global infections. Whether these tools can be used in the same way to study other subtypes is unknown. Several studies suggest that all HIV-1 subtypes are likely to be similarly sensitive to ARVs19,20, but there is increasing evidence that subtypes under selective pressure have different evolutionary pathways leading to HIVDR21. A high proportion of viruses from our study population were BF recombinant forms, known to be circulating in Argentina since the early 1980s22,23. Despite their great intrinsic genetic variability6, the polPR-RT gene region shows highly conserved subtype F genomic segments at codons where the frequency of some RAMs differed significantly between B and BF recombinants.
The I54V mutation, associated with high resistance to all PIs except darunavir24, was more frequent in BF recombinants, while the K103S mutation, associated with high resistance to nevirapine and efavirenz25, was more frequent in subtype B sequences. The K103S mutation results from a double base change at codon 103 of TR and typically develops from pre-existing K103N mutations26. Furthermore, high-resistance TAM1 profile mutations, associated with zidovudine and stavudine, were more prevalent in BF recombinants than in subtype B sequences.
There are several reasons that could explain the differences in the selection of RAMs between subtypes. Differences in the genetic barrier could be involved; such has been observed in the V106M mutation, which is more common in patients with subtype C because a single transition is required for its appearance, while in other subtypes two transitions are required27.
On the other hand, different fitness, adaptability, or structural barriers given by the characteristic genetic context of each subtype could also be involved28,29. The use of codons between the B and BF subtypes in our population was evaluated; however, no correlation was found between the genetic barrier and the observed changes. It is possible that the differences between the subtypes are mainly related to viral fitness. Importantly, it is unlikely that HIV-1 subtype-specific mutations affect the efficacy of ART, at least with current treatment regimens.
Acquired resistance and virologic failure have declined in children and adolescents with HIV and virologic failure over the past 15 years, likely due to continued improvement in the efficacy and potency of ART regimens and changes in criteria for virologic failure. However, HIV-1 resistance to ARVs remains a challenge for patients with poor treatment adherence and multiple virologic failures. In addition, there are differences in resistance patterns between subtypes B and BF recombinants. Whether these can impact future ART effectiveness is unknown.











 nueva página del texto (beta)
nueva página del texto (beta)