INTRODUCTION
Iron overload (IO) is a clinical condition that may be caused by increased iron absorption (i.e., hereditary hemochromatosis [HHC]) or secondary to blood transfusions due to various hematological disorders1. IO is found in up to 78% of patients with chronic liver disease (CLD) of any nature2,3, being more frequent in patients with hepatitis C virus (HCV) infection4. In patients with CLD, local iron accumulation occurs secondary to local iron deposits and hepcidin dysregulation4,5.
Even though IO is associated with reduced early survival after liver transplantation (LT) in patients with HHC, long-term follow-up studies have revealed that after several months, LT normalizes serum hepcidin levels and prevents hepatic IO recurrence6, attributing the dismal outcomes to extrahepatic IO and other causes7. In people without HHC, previous data have suggested that survival may be reduced in patients with IO who underwent an LT8.
Regarding LT complications, IO has been associated with invasive fungal infections and disseminated disease from opportunistic pathogens, even in patients without HHC9-11. In addition, in the context of hematopoietic cell transplantation, a therapy involving high immunosuppression, IO has also been associated with a higher risk of infections and a lower risk of non-relapse mortality within the first 90-day post-hematopoietic cell transplantation12,13.
Currently the effect of IO in patients without HHC submitted to LT remains to be elucidated. This study aims to determine the clinical impact of IO, diagnosed by histopathology, in patients who underwent LT at a tertiary referral center in Mexico City.
MATERIALS AND METHODS
Study population
We conducted a retrospective cohort study that included all patients over 18 years undergoing LT at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán over 3 years, from 2015 to 2017. We excluded patients diagnosed with HHC or those missing crucial data (such as information regarding liver explant or follow-up outcomes from electronic and physical records). All LTs performed at our institution were conducted via controlled graft donation after cardiac death.
Basal characteristics and pretransplant evaluation
Baseline and transplant-related characteristics were collected from clinical records. Etiology was grouped into four categories: autoimmune, viral, and metabolic (which included alcohol and metabolic-associated liver disease), and others. Immunosuppressive therapy included basiliximab and methylprednisolone as induction, followed by calcineurin inhibitors (cyclosporine A or tacrolimus), steroid therapy (methylprednisolone and prednisolone), or mycophenolate mofetil. Antifungal prophylaxis consisted of intravenous anidulafungin 100 mg before LT, when appropriate. All patients had cytomegalovirus IgG status determined as part of the pretransplant evaluation, whose management was guided by the center's cytomegalovirus disease prevention protocol, reported previously14, assuming all donors were positive for local protocol purposes. For the dynamic profile of liver function tests, laboratory variables were analyzed immediately before LT and at 7, 30, and 90 days after LT. The length of the intensive care unit and hospital stay was calculated from the date of LT to the intensive care unit and hospital discharge.
Liver biopsies and iron overload
The stored explant liver biopsies embedded in paraffin were reprocessed and stained using Perls Prussian blue stain. Two expert pathologists analyzed the liver biopsies, masked to the patient's clinical data and each other's results. As reported previously by Deugnier et al.15,16, iron deposits were assessed by evaluating the size and location in the hepatocytes and the sinusoidal and portal areas. These results were multiplied by a coefficient of 3, as Brissot et al.17,18 recommended, obtaining the hepatocyte, sinusoidal, and portal iron scores. These individual scores completed the total iron score, divided by age, to obtain the histochemical hepatic iron index (HHII), considering an HHII score ≥ 0.15 as IO. The cutoff was extrapolated from the study's data by Deugnier et al., which concluded that such cutoff easily differentiated between homozygotes and heterozygotes in HHC and that the HHII can be used as an alternative to the biochemical hepatic iron index15. Patients classified differently by the two pathologists were analyzed and organized in a third joint revision.
Outcomes
The outcomes of interest were time from LT to death, rejection, infection, readmission, and complication. For rejection, the date taken was that of the liver biopsy. A liver biopsy for prompt evaluation was obtained if the liver function tests showed significant alterations or if there was clinical suspicion of liver failure. The dates of infection, readmission, and complication dates were those when they were first documented. International guidelines defined infection as systemic inflammatory response syndrome with microbiological isolation in cultures or as sepsis19. Infections were classified as bacterial, fungal, and combined or unknown. Complications were divided into three groups: mechanic, metabolic, and both. Mechanic complications were categorized as biliary, vascular, or both; metabolic complications were classified as renal, cardiovascular, and others, which included endocrinological, neurological, pulmonary, and others. Only events within 90 days after LT were considered, based on the Clavien-Dindo classification of surgical complications20 and previous data reporting normalization of iron metabolism alterations after LT6.
Statistical analysis
Data were reported with median and interquartile range, mean with standard deviation if numerical, and frequency and percentages if categorical. Baseline characteristics were compared between patients with and without IO, using a t-test that allows for heteroscedasticity when numerical or a Chi-squared test when categorical. The interobserver reliability of the gold standard for IO was measured using the kappa index. The dynamic profile of each liver function test was compared between patients with and without IO to assess the impact of IO, using linear mixed effect models that controlled for age, etiology, and the baseline value of the corresponding liver function test. The survival within the first 90 days after LT was estimated using the Kaplan–Meier method and compared between subjects with and without IO using the log-rank test (unadjusted comparison). The impact of IO on survival was further assessed using a Cox proportional hazards model that controlled for age and etiology (categorized as autoimmune, viral, or metabolic/others), and another Cox model analysis that controlled for age, etiology, diabetes, alcohol use, and obesity. The proportional hazard assumption was verified using the Schoenfeld residuals. Similar survival analyses were performed to determine the impact of IO on the risk of rejection, infection, readmission, and complication in the first 90 days after LT. For such analyses, death was considered a censoring event, and it was assumed to be non-informative for the events of complication, infection, and readmission. The significant level was established as 0.05 at two-tails. Data were analyzed using the free software R, version 4.1.2.
Human subjects
All research was conducted following both the Declarations of Helsinki and Istanbul. The Institution's Ethics and Clinical Investigation committees approved the study, reference number 2783, and the National Commission of Bioethics of Mexico, registration number 09-CEI-011-20160627. The Institutional Review Board waived the informed consent since this study did not require the patient to undergo additional procedures.
RESULTS
Baseline characteristics and reliability of the Gold Standard
During 2015-2017, 117 patients underwent LT, of whom only 105 had a stored explant liver biopsy that could be processed. Forty-seven patients were found with IO (45%), predominantly men (n = 27/47, 57%). Regarding the cause of cirrhosis, viral and metabolic etiologies were more frequent in patients with IO than in patients without IO (viral etiology in 43% vs. 21%, and metabolic etiology in 32% vs. 22%, p = 0.011). All patients in the viral etiology group had HCV infections. The metabolic group comprised patients with metabolic-associated fatty liver disease (11 patients), cryptogenic disease (15 patients), alcohol-related liver disease, and lysosomal acid lipase deficiency (one each). The "Other" group included the remaining patients (n = 9) due to considerable differences in physiopathology: three patients underwent an LT due to bile duct injury, and one each due to transthyretin amyloidosis, idiopathic fulminant hepatitis, biliary atresia, biliary cyst, congenital hepatic fibrosis, and drug-induced hepatitis. Comparing comorbidities between patients with and without IO, patients with IO had a higher prevalence of obesity (32% vs. 12%, p = 0.013), alcohol consumption (34% vs. 9%, p = 0.001), and type 2 diabetes (T2D) (28% vs. 12%, p = 0.043). Table 1 describes additional baseline clinical and laboratory characteristics. It is essential to note the higher MELD (median 18 vs. 16, p = 0.0018) and MELD-Na (median 22 vs. 18, p = 0.007) scores in patients with IO compared to those without IO. Confirmation of hepatocarcinoma in the liver explant was reported in 23% of the patients (n = 24/105), mainly presented in the viral etiology group (13 patients) and the metabolic etiology group (nine patients). Concerning the reliability of the gold standard for IO, the histochemical evaluation of both pathologists was concordant in 94% of the biopsies (a total of 99 biopsies, 56 with IO, and 45 without IO), with a Kappa index of 0.92 (95% confidence interval (CI), 0.85-0.99).
Table 1. Basal clinical and laboratory characteristics
| Variables | Iron overload group (n = 47) |
Normal iron levels groups (n = 58) |
p-value¶ |
|---|---|---|---|
| Gender* | |||
| Male | 27 (57%) | 23 (40%) | 0.070 |
| Female | 20 (43%) | 35 (60%) | |
| Age, years‡ | 55 (46-59) | 50 (39-58) | 0.2 |
| Etiology* | |||
| Autoimmune | 10 (21%) | 26 (45%) | 0.011 |
| Viral | 20 (43%) | 12 (21%) | |
| Metabolic | 15 (32%) | 13 (22%) | |
| Other | 2 (4%) | 7 (12%) | |
| Comorbidities* | |||
| Obesity | 15 (32%) | 7 (12%) | 0.013 |
| Alcohol consumption | 16 (34%) | 5 (9%) | 0.001 |
| T2D | 13 (28%) | 7 (12%) | 0.043 |
| HTN | 7 (15%) | 7 (12%) | 0.7 |
| Autoimmune diseases | 6 (13%) | 9 (16%) | 0.7 |
| Immunosuppressive treatment* | |||
| Basiliximab | 41 (87%) | 49 (84%) | 0.9 |
| Calcineurin inhibitors | 47 (100%) | 56 (97%) | 0.5 |
| Steroid | 47 (100%) | 57 (98%) | 0.5 |
| MMF | 41 (87%) | 48 (84%) | 0.7 |
| Liver function tests‡ | |||
| Total bilirubin, mg/dL | 5.6 (2.9-8.4) | 4.1 (1.9-9.7) | 0.13 |
| Direct bilirubin, mg/dL | 2.5 (1.2-4.8) | 1.6 (0.7-5.8) | 0.3 |
| Indirect bilirubin, mg/dL | 2.92 (1.9-4.2) | 2.2 (1.2-4.1) | 0.066 |
| ALT, U/L | 35 (18-64) | 48 (25-72) | 0.14 |
| AST, U/L | 65 (36-105) | 68 (44-108) | 0.6 |
| ALP, U/L | 173 (114-238) | 191 (126-296) | 0.079 |
| GGT, U/L | 44 (30-88) | 87 (47-132) | 0.015 |
| Albumin, g/dL | 2.8 (2.3-3.45) | 3.1 (2.6-3.5) | 0.2 |
| Complete blood count‡ | |||
| Leukocyte count, × 103/µL | 4.4 (3.3-5.6) | 4.3 (2.9-5.5) | 0.6 |
| Absolute neutrophil count, × 103/µL | 2.9 (2.1-3.7) | 2.7 (1.8-3.7) | 0.8 |
| Hemoglobin§, g/dL | 11.6 (2.6) | 11.6 (2.2) | 0.9 |
| Platelet count, × 103/µL | 66 (47.5-88) | 66 (50-110.7) | 0.4 |
| Clinical variables‡ | |||
| Previously transfused patients | 9 (18%) | 10 (17) | 0.810 |
| MELD | 18 (14-22) | 16 (12-19) | 0.018 |
| MELD-Na | 22 (18-25) | 18 (11-22) | 0.007 |
| Anidulafungin prophylaxis | 6 (13%) | 10 (18%) | 0.5 |
*n (%).
‡Median (IQR).
§Mean (SD).
¶Pearson's Chi-squared test; Fisher's exact test; Wilcoxon rank sum test; Welch two-sample t-test.
ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyl transferase; HTN: primary hypertension; MMF: mycophenolate mofetil; T2D: type 2 diabetes; w/IO: with iron overload; w/o IO: without iron overload.
Dynamic profile of liver function tests
The dynamic profile of the liver function test levels by IO status is displayed in figure 1. Overall, the rate of change over time was similar between patients with and without IO (p > 0.1 for all tests). The mean ALT and GGT levels increased in the first-week post-LT and decreased afterward, while the mean albumin levels had the opposite behavior. The mean level of the remaining liver function tests decreased over time, with the highest decline during the first week.
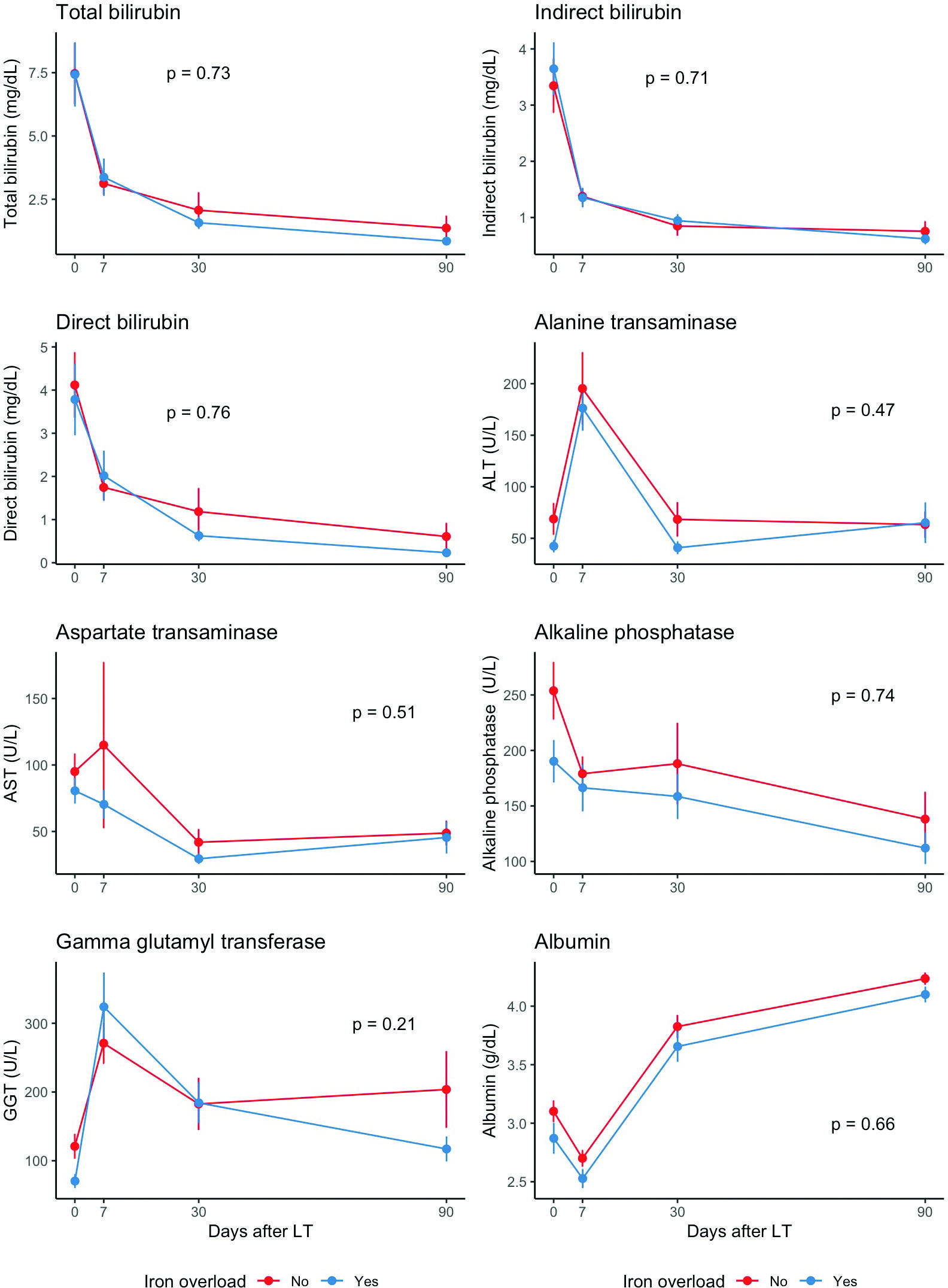
ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyl transferase; LT: liver transplant.
Figure 1. Longitudinal analysis for liver function tests comparing iron overload status. A: total bilirubin; B: indirect bilirubin; C: direct bilirubin; D: alanine transaminase; E: aspartate transaminase; F: alkaline phosphatase; G: gamma-glutamyl transferase; H: albumin.
Outcomes
The incidence rates within 90 days of the primary outcomes are summarized in table 2. Compared to patients without IO within 90 days, the death incidence rate tended to be lower in patients with IO (0.7 vs. 4.5 deaths/100 person-months, p = 0.055), while the incidence rate of any complication (mechanic, metabolic, or both) was significantly higher in patients with IO (223 vs. 93 complications/100 person-months, p = 0.043). When analyzing the specific complications, we found no difference between any metabolic complications (74.4% vs. 60%). We found no differences in the incidence rates within 90 days of rejection, infection, and readmission, as well as in the patient's length of stay and the intensive care unit stay.
Table 2. Outcomes in the first 90 days post-transplantation
| Outcome | With IO (n = 47) |
Without IO (n = 58) |
p-value* |
|---|---|---|---|
| Incidence rate within 90 days (events/100 person-months) |
Incidence rate within 90 days (events/100 person-months) |
||
| Death | 0.7 | 4.5 | 0.055 |
| Rejection | 2.3 | 2.7 | 0.89 |
| Infection | 28.7 | 27.0 | 0.81 |
| Readmission | 6.5 | 9.6 | 0.33 |
| Complication | 223.0 | 92.8 | 0.043 |
| Median (IQR) | Median (IQR) | ||
| Length of hospital stay, days | 13 (9-19) | 11 (9-16) | 0.5 |
| Intensive care unit, days | 4 (3-5) | 3.5 (3-6) | 0.9 |
*Log-rank test, Chi-squared test.
IO: iron overload.
The event-free survival curves for death, rejection, infection, readmission, and complication, stratified by IO status, are displayed in figure 2; concerning the median time to the primary outcomes, only the time to any complication reached its median with a 95% CI. This median time to a complication was lower in patients with IO (median of 2 days vs. 3 days).

LT: liver transplant.
Figure 2. Kaplan-Meier event-free survival curves for the primary outcomes stratified by iron overload status. A: overall survival; B: rejection-free survival; C: infection-free. D: readmission free-survival; E: complication free-survival.
Table 3 shows the hazard ratios (HR) (unadjusted, adjusted for age and etiology, and adjusted for age, etiology, diabetes, alcohol use, and obesity) for the primary outcomes during follow-up within the first 90 days after LT. In the unadjusted and adjusted for age and etiology analysis, the only differences found were a trend toward a lower hazard of death in patients with IO and a trend toward a higher risk of complications in patients with IO. Concerning the adjusted analysis for age, etiology, alcohol use, and obesity, the presence of IO was associated with a lower risk for death (adjusted HR = 0.09, CI 95% = 0.01-0.83, p = 0.033) and a higher risk of complications (adjusted HR = 1.70, CI 95% = 0.02-2.83, p = 0.042). Eight patients died during the first 90 days after LT, corresponding to 8% of the total cohort, and only one had IO. Five died from infections: three due to invasive fungal infections, one due to invasive bacterial infection from Klebsiella oxytoca, and one due to septic shock without microbiological isolation. Two died from bleeding: One from subarachnoid hemorrhage and one from hemorrhagic shock due to a tear in the mesenteric vein after LT. The remaining patient died from a massive pulmonary embolism. Of the patients who died, all had at least one type of complication; two were readmitted, and only one was reported with rejection.
Table 3. Hazard ratios of specific outcomes for iron overload versus no iron overload
| Outcome | Unadjusted | Adjusted for age and etiology* | Adjusted for age, etiology*, diabetes, alcohol use, and obesity‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | CI 95% | p-value | HR | CI 95% | p-value | HR | CI 95% | p-value | |
| Death | 0.17 | 0.02-1.35 | 0.092 | 0.13 | 0.02-1.06 | 0.057 | 0.09 | 0.01-0.83 | 0.033 |
| Rejection | 0.90 | 0.20-4.00 | 0.89 | 1.77 | 0.34-9.21 | 0.50 | 1.59 | 0.28-9.10 | 0.60 |
| Infection | 1.03 | 0.59-1.79 | 0.93 | 1.21 | 0.66-2.20 | 0.54 | 1.26 | 0.66-2.42 | 0.49 |
| Readmission | 0.68 | 0.28-1.64 | 0.39 | 0.60 | 0.24-1.51 | 0.28 | 0.54 | 0.20-1.46 | 0.23 |
| Complication | 1.51 | 0.99-2.31 | 0.056 | 1.55 | 0.99-2.42 | 0.053 | 1.70 | 1.02-2.83 | 0.042 |
*For these analyses, etiology was modified into three categories: autoimmune, viral, metabolic, and others.
‡The analysis for rejection did not adjust for obesity due to the lack of events in some strata defined by obesity and iron overload.
HR: hazard ratio; CI: confidence interval.
DISCUSSION
Our study at a tertiary referral center in Mexico City found that patients with IO and CLD were predominantly males and had more frequent viral and metabolic etiologies. IO was associated with obesity, alcohol consumption, and T2D. In addition, patients with IO had both a higher rate of complications and a higher overall survival (OS) than those without IO.
Consistent with previous data, we found a high IO prevalence (44.7%, n = 47/105) in patients submitted to LT. Compared to the first reports of patients with HHC, a similar prevalence (31.8%, n = 7/22) of IO was found in the study by Crawford et al.21. In addition, the investigation by Kowdley et al. reported similar data, reporting a hepatic iron index ≥ 1.9 (calculated as the division of hepatic iron concentration (HIC), measured in µmol/g, by age) in 50.8% (n = 100/197) of their population8. When analyzing the information available in the non-HHC patients submitted to LT, a similar frequency was reported in the study by Alexander et al., with the presence of IO in 31.3% (n = 48/153) of their patients by histological evidence11.
Regarding the baseline characteristics of our patients, our results are coherent with previous information that mentioned the high frequency of men among patients with IO (57%, n = 27/47). Similar information was detailed in the study by Alexander et al., where 66.6% (n = 32/48) of the patients with IO were male11, as well as in the study by Stuart et al., where a univariate analysis associated an increased hepatic iron concentration with men that underwent LT22. In Mexico, a lower MELD score in patients who underwent LT was found previously by Vilatobá et al.23, who reported data from our same center although in different periods. Another study from our country, conducted in the city of Monterrey, also reported a lower MELD score in similar patients submitted to LT24 without explaining such differences compared to other countries. Regarding the higher MELD score in our patients with IO, similar results were reported by Fierro-Fine et al., who also found a higher MELD score in the heavy iron group25.
IO has been associated with viral etiologies of CLD, particularly HCV infection26,27. A relationship between low serum hepcidin levels and IO has been suggested. Hepcidin is directly suppressed by HCV and diminished in CLD states4; it has been noted that such alterations may normalize after LT4,6, which led us to analyze only early complications after LT. In our study, HCV infection in the group with IO was the most frequent cause of CLD, affecting 43% of the patients (n = 20/47). Such frequency was consistent with the information reported by Alexander et al., where HCV infection was present in 47.1% (n = 72/153) of the CLD cases and higher than the data reported by Stuart et al., where 17.3% (n = 18/104) of the patients with IO had CLD due to HCV infection. The contrasts between studies may be due to differences in the global distribution of HCV28 and the local institution transplant protocols.
Our study found that obesity, alcohol consumption, and T2D were associated with IO in patients with CLD who underwent LT. Several comorbidities have been reported previously in patients submitted to LT in distinct cohorts. As an exploratory analysis, we evaluated potential predictors for IO among basal characteristics using a univariate logistic regression and then analyzed statistically significant variables with a multivariate logistic regression model. The results suggest that obesity (prevalence odds ratio 4.35, 95% CI = 1.52-12.45, p = 0.006) and alcohol consumption (prevalence odds ratio 6.68, 95% CI 2.15-20.82, p = 0.001) were associated with IO. Compared to the data reported by Crawford et al., where 50% (n = 11/22) of their entire cohort reported alcohol consumption of at least 60 g/day21, we found a lower occurrence of alcohol consumption in our patients, stated in 20% (n = 21/105) of the patients and of 34% (n = 16/47) in the group with IO. In addition, we found a higher prevalence of T2D in our patients when compared to the study by Alexander et al. and lower versus the study by Crawford et al.; we documented the presence of T2D in 27.6% (n = 13/47) of the patients with IO, versus 16.6% (n = 8/48) in the study conducted at the University of Washington11, and 40.9% (n = 9/22) in the United Kingdom cohort of patients with HHC. These different results could be explained by the higher prevalence of T2D in Mexico when compared to the United States (16.8% in 2018 vs. 14.3% in 2017-18, respectively)29,30 and the higher frequency of metabolic-associated fatty liver disease and metabolic-associated fatty liver disease induced CLD in the Mexican population31,32. Moreover, an elevated frequency of T2D in patients with HHC has been reported, ranging from 20% to 50%, depending on the source33.
In patients with IO due to hematological diseases (i.e., thalassemia syndromes, sickle cell disease, and other anemias), ferritin has been unable to predict changes in HIC34. On the contrary, it has been suggested that people with CLD and elevated ferritin (above 300 µg/L in males and 200 µg/L in females) and transferrin saturation (Tsat; above 50% in males, and 40% in females) should prompt the investigation of HHC and HFE genotyping, representing an alteration in HIC35. The IO status in patients with CLD has been attributed to various causes, mainly liver necrosis and chronic viral hepatitis36. Considering such information, as an exploratory analysis, we studied the median Tsat and ferritin data available in 43 of our patients (17 with IO and 26 without IO), solicited as etiology work-up, because of anemia without clear signs of bleeding, or as a pretransplant evaluation. We found noticeable differences in the median Tsat and ferritin despite the small number of patients, well above the reported thresholds in patients with IO by histopathology (median Tsat of 74% and median ferritin of 346 ng/mL in the group with IO versus a median Tsat of 25% and median ferritin of 61 ng/mL in the group without IO, p < 0.001). When comparing these results to the available data of the non-HHC patients, we found results similar to those of Stuart et al., where serum ferritin and Tsat were augmented with HIC22. In addition, when comparing the cutoff mentioned above for ferritin and Tsat to the histopathological diagnosis of IO in our patients, combined serum iron tests combined yielded a sensitivity of 81% (95% CI = 64-98%) and a specificity of 69% (95% CI = 51-87%).
Different complications after LT have been reported in patients with IO, with and without HHC, mainly due to invasive infections by bacteria and fungi, cardiovascular disorders, and death8,11,21,37. In our study, we found a higher incidence of complications in patients with IO, without differences between the subtypes of complications (mechanic or metabolic), in the incidence of infection (both groups had an infection rate of almost 30 infections/100 person-months), or in the microorganism causing the infection (bacterial or fungal). No data have been found regarding post-operative complications in patients who underwent LT. The only available data regarding IO and post-operative complications come from the analysis by Gerhard et al., who found similar results. This prospective cohort evaluated patients submitted to Roux-en-Y gastric bypass who underwent an intraoperative liver biopsy to detect hepatic iron staining. They did not find significant differences in major or minor complications compared to hepatic iron status, only a higher rate of minor complications attributed to higher Tsat levels38.
Several reasons may account for the notorious differences in infection rates. First, the monoclonal antibody directed at CD3 OKT3, which has long been associated with increased bacterial and fungal infections during the 1st month after LT9,39,40, was not used in our patients. Second, differences in time may account for better pre-transplant evaluation, as seen in the use of anidulafungin as fungal prophylaxis in selected patients, compared to the oral nystatin used in the study by Alexander et al.11. Finally, the low frequency of septic shock in our patients probably represents a low rate of invasive infections, avoiding the dismal outcomes in the presence of such complications.
Our OS results differed from previous HHC and non-HHC cohorts, albeit, like the data published by Stuart et al. Concerning these variations, the difference in the diseases for which the LT was indicated between our cohort and the HHC cohorts is to be considered since various reports have stated lower survivals in patients with HHC after LT8,21. Second, the low rate of invasive infections, particularly in the IO group, could account for the low mortality, as only one patient with IO died within 90 days after LT. Compared to the study by Alexander et al., which mainly reflects our type of study, the main differences to be considered are the immunosuppressive regimes and the standard of care that have changed throughout time, benefiting the patients even in the presence of IO.
Interestingly, we found in our cohort the same behavior in patients with IO in our cohort as the one reported by Stuart et al., where the patients with IO had longer OS, statistically significant after controlling for age, etiology, diabetes, alcohol use, and obesity. One and 5-year OS was 80% and 82% in the group with IO, compared to 71% and 75% in the group without IO, respectively 22. In our cohort, the 1-year and 5-year OS were 96% and 89% in patients with IO and 88% and 82% in patients without IO, respectively. The reason for such clinical behavior has yet to be clarified and deserves further exploration in future prospective studies.
Our study has several limitations besides the inherent ones to the retrospective nature of the study. First, information comes from a single referral center; therefore, our cohort does not represent the Mexican population. Second, for budgetary reasons, in our institution, we do not investigate the presence of HHC nor perform HFE genotyping or measure the HIC (in µmol/g). Third, in our cohort, death was a competing event for infection, rejection, and readmission. The survival analyses for those outcomes rely on the assumption that censoring due to death is not informative, an assumption that cannot be verified. However, we preferred to make this assumption instead of performing a competing risk analysis because the sample size is small, and the results from those analyses are hard to interpret. Fourth, rejection events might have been missed in patients who died because autopsies are not a rule in our center (only one rejection was documented in the 8 patients who died within 90 days after LT). Results regarding this outcome should be interpreted more carefully.
In conclusion, detecting iron overload in patients with chronic liver disease during the evaluation for orthotopic liver transplantation is essential to identify the patients at risk of early complications. Our study did not reproduce the negative impact of iron overload on survival, even reporting a prolonged survival in such patients. Based on our findings, we do not recommend any intervention, such as iron chelation therapy, but support the need for closer surveillance in patients with concomitant iron overload undergoing liver transplantation. Further studies are required to understand the role of IO on survival.











 nueva página del texto (beta)
nueva página del texto (beta)


