INTRODUCTION
Tobacco smoking is the leading cause of preventable disease, disability, and death in the world. Over 80% of the 1.3 billion tobacco users worldwide live in low- and middle-income countries, where the burden of tobacco-related illness and death is the heaviest1. Recognizing tobacco use as an addiction is critical both for treating the tobacco user and understanding why people continue to use tobacco despite the known health risks.
The central element among all forms of drug addiction is that the user's behavior is largely controlled by a psychoactive substance (i.e., a substance that produces transient alterations in mood primarily mediated by brain effects). Nicotine is a powerful reinforcer, that is, the pharmacologic activity of the drug is sufficiently rewarding to trigger and maintain self-administration in a majority of regular users2.
All tobacco products contain substantial amounts of nicotine and other alkaloids. Nicotine is a tertiary amine composed of a pyridine and a pyrrolidine ring. Nicotine may exist in two different three-dimensional structured shapes called stereoisomers. Tobacco contains only (S)-nicotine (also called 1-nicotine), which is the most pharmacologically active form. Tobacco smoke also contains the less potent (R)-nicotine (also called d-nicotine) in quantities of up to 10 percent of the total. The physical characteristics of nicotine delivery systems can affect their toxicity and addictiveness. Therefore, the evaluation of new electronic nicotine delivery systems (ENDS) should consider their toxic and addictive effects2. The ENDS such as e-cigarettes and heated tobacco products are novel battery-operated devices that deliver nicotine without the combustion of tobacco. The use of ENDS is sometimes referred to as "vaping." A typical EVP device contains three main components: a battery, a heating element, and a cartridge or tank that holds the e-liquid. When a user takes a puff from an EVP, the e-liquid is heated by the heating element and forms particles and gases that the user inhales into their lungs.
The use of e-cigarettes has increased considerably among young people, and the predominant dual use of tobacco and electronic cigarettes has been documented. For example, e-cigarette use has been associated with other tobacco products among youth and young adults, including combustible tobacco3,4. The main impact is nicotine addiction, with tobacco administered through different vehicles.
Findings released from the most recent Monitoring the Future survey of substance use behaviors and related attitudes among teens in the United States indicate that from 2017 to 2019, the percentage of teenagers who said they vaped nicotine in the past 12 months roughly doubled for 8th graders from 7.5% to 16.5%, for 10th graders from 15.8% to 30.7%, and for 12th graders from 18.8% to 35.3%. In 2020, the rates held steady at 16.6%, 30.7%, and 34.5%, respectively5. There is an urgent need for better evaluation and intervention in e-cigarette users to predict and mitigate the possible health risks, particularly because the potential risks remain uncertain and partially undefined.
Notably, the current paradigm for smoking cessation conceptualizes nicotine addiction as a chronic, relapsing disorder that benefits from long-term management and intensive treatment approaches, as do other chronic diseases6. Efforts to reduce tobacco and nicotine use in our society must address all the major influences that encourage continued use, including social, psychological, and pharmacologic factors. Therefore, the present review aims to describe addiction to tobacco smoking and vaping.
MATERIALS AND METHODS
Search strategy
This narrative review was performed by collecting clinical trials, basic research, and reviews. The Cochrane rapid reviews guidelines were used as the search framework7. We researched within the following databases: PubMed, PsycINFO, PsycARTICLES, and Cochrane Clinical Trials Library using the keywords, or combination of keywords: "smoking," "tobacco," "e-cigarette," "vaping," or "ENDS" and "smoking-vaping cessation." In addition, articles published in peer-reviewed scientific journals, WHO reports, and informs of the Surgeon General on Tobacco were included.
NICOTINE IS A POWERFULLY ADDICTING DRUG: NEUROPHYSIOLOGY OF NICOTINE ADDICTION AND MECHANISMS OF ACTION OF DRUGS IN NICOTINE ADDICTION
Nicotine is an alkaloid from the tobacco plant and its main psychoactive substance. Nicotine binds to cholinergic receptors and generates neuroadaptations that can trigger the addiction cycle.
There are several types of nicotinic cholinergic receptors, all of 5 units that are arranged symmetrically around a central pore. Each subunit comprises four transmembrane domains with the N-and C-termini located extracellularly. Subtypes of neuronal receptors may be formed of a single type of subunit (homomeric) or have combinations of α and β subunits (heteromeric)8. Different types of nicotinic cholinergic receptors have a role in addiction such as α7 and α9 homomeric receptors, as well as α3β4 and α7β2 heteromeric receptors. The subtype α4β2 receptors play an important role in addiction due to the affinity of nicotine and the effect their stimulation has on dopamine release9.
One of the mechanisms implicated in the addictiveness of nicotine is the release of dopamine in the brain's reward circuit. Nicotine activates the mesocortical and mesolimbic dopaminergic pathways, releasing dopamine and noradrenaline10,11. These pathways include the ventral tegmental area (VTA), and the nucleus accumbens (NA) and extend to limbic structures (ventral striatum, hippocampus, and amygdala) and the cerebral cortex (frontal, cingulate, and entorhinal cortex). This activation triggers reward learning mechanisms that create conditional associations between the pleasurable sensation of nicotine consumption, the smoking behavior itself, and the universe of related cues that help explain the compulsive nature of repeated nicotine use. However, not just the reward learning response initiates and maintains the addiction. Dopamine is also implicated in the modulation of aversive responses, novelty seeking, expectation, prediction of errors, decision making, and, in general, information processing of the consumer's environment10,11.
A comprehensive and integrative model of nicotine addiction should consider the different dimensions of nicotine in cognition, emotion, and behavior. Nicotine potentiates cognitive functions due to its action on the cortex, frontal and parietal areas, the anterior cingulate, and the superior colliculus. This effect has been appreciated, particularly in those with nicotine dependency, who receive nicotine after a deprivation period12.
Pharmacotherapies for smoking cessation act on the cholinergic or dopaminergic systems and can prevent or diminish withdrawal symptoms. They may act in 3 ways: inhibition of dopamine and norepinephrine reuptake, blockade and partial agonism of α4β2 nicotine receptors, and substitution of nicotine from tobacco. Nicotine replacement therapy (NRT) includes several pharmaceutical products that extensively bind to nicotinic cholinergic receptors without producing the same rewarding effects of smoked nicotine. Varenicline is a partial agonist at a specific nicotinic receptor subtype composed of α4β2 subunits located in the dopaminergic neurons of the VTA. Bupropion is a non-nicotinic medication that inhibits dopamine and norepinephrine reuptake (Fig. 1). Nicotine stimulates the nicotinic cholinergic receptors of the NA and the VTA, activating the mesolimbic pathway and mesocortical pathways. The psychoactive effects of nicotine depend on dopaminergic and glutamatergic systems activation
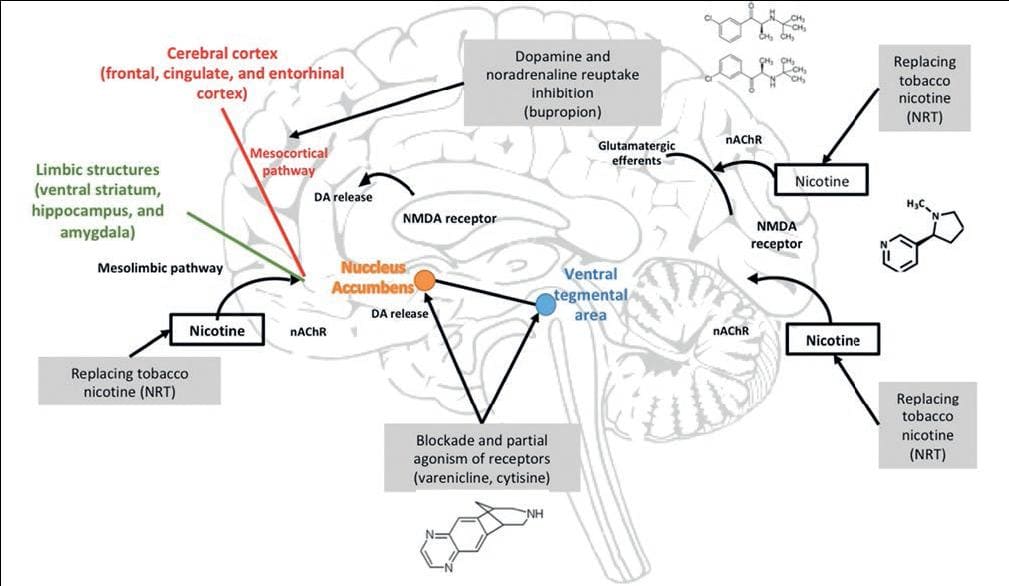
Figure 1. Neurophysiological mechanisms of nicotine action. DA: dopamine; nAChR: nicotine acetylcholine receptor; NMDA: N-methyl-D-aspartate; NRT: nicotine replacement therapy.
Nicotinic receptors influence the release of multiple neurotransmitters such as dopamine, noradrenaline, acetylcholine, glutamate, and GABA. As a result, nicotine disrupts multiple neurotransmitter systems in the brain. Besides, cholinergic receptors are widely distributed in the central and peripheral nervous system. Both nicotinic and cholinergic muscarinic receptors fulfill functions in the autonomic nervous system, essential for maintaining homeostasis in the circulatory, respiratory, integumentary, digestive, urinary, and neuronal brain systems13.
Exposure to nicotine can cause developmental disorders and diseases at different stages of life (Table 1).
Table 1. Health risks derived directly from nicotine addiction according to study type
| Health effect | Human studies | Animal studies |
|---|---|---|
| Cardiovascular stimulation with low doses | Vasoconstriction, increase in blood pressure and heart rate. Increase tone and motor activity of the GI tract52. Upregulation of nicotine receptors. | |
| Nicotine intoxication* (with e-liquids, mainly in young children)45 | Mortal dose in adults around 60 mg (total content of 20 cigarettes between 120-220 mg, 20-60 mg absorbed by smoking). Nausea, vomit, diarrhea, sweating, salivation, abdominal pain, weakness due to neuromuscular blockade, stimulation of varied sensorial receptors, tremor, mental confusion, convulsions, stimulation of CNS followed by depression and respiratory failure. | |
| Carcinogenesis | No causal evidence46. | No causal evidence. However, in vivo and in vitro studies support enhanced carcinogenesis of cancer cells exposed to nicotine47. |
| Fertility and reproduction | Infertile smoking subjects had significant increase in sperm fragmentation, caspase-3, and cotinine but reduced motility, morphology, and pH of semen more marked with higher cotinine level48. | Decreased spermatic count and sperm motility49. |
| Development | Exposure during fetal life to smoking mothers associated with orofacial clefts, preterm birth, stillbirth, intrauterine growth restriction, neurodevelopment, and behavior alterations33,34. | Autonomic abnormalities in serotonin and
norepinephrine pathways in the fetal brain50. Decreased cognitive development51. |
| Cardiovascular | Exposure during fetal life to smoking mothers associated with cardiovascular abnormalities, poor lung growth, wheezing, and airflow obstruction. Nicotine is easily absorbed and crosses the placenta with levels in fetal blood similar to those of the mother33,34. | Increased blood pressure, dyslipidemia, endothelial dysfunction, and myocardial remodeling53. |
*Requires gastric lavage, activated carbon and support.
Besides, it is important to consider other targets and mechanisms involved in addiction. For example, it is known that the nicotinic cholinergic receptors become desensitized after one or two cigarettes. Consumption of a higher dose of nicotine no longer activates the mesocorticolimbic dopaminergic pathway, so it is necessary to review the mechanisms of other neurotransmitters10.
For instance, metabotropic glutamate receptors of the subtype 5 (mGluR5) interact with N-methyl-D-aspartate (NMDA) and dopamine D2 receptors (DRD2), mediating the reinforcing and addictive properties of nicotine. Particularly, mGluR5 antagonists reduce the nicotine-induced dopamine release in the NA and diminish nicotine self-administration in rodents14.
As to other neurotransmitters, serotonin 5HT2C receptors are expressed on the VTA, NAc, and prefrontal cortex (PFC). In the VTA, 5-HT2C receptors are located on γ-aminobutyric acid (GABA) inhibitory neurons. Activation of 5-HT2C receptors on GABAergic neurons stimulates GABA release, which decreases the firing of postsynaptic dopaminergic neurons. This inhibition reduces dopamine release in the mesolimbic pathway, the NAc, and the PFC, resulting in a lower reward response15. Lorcaserin is a 5-HT2C receptor agonist used to decrease appetite in treating obesity. In addition, it has been shown to reduce the reinforcing effects of misused drugs. Studies have shown that lorcaserin reduces the self-administration of nicotine, cocaine, and opioids in rat models, and reduces alcohol intake in alcohol-preferring rats, particularly, combining when lorcaserin with NRT16.
CONTRIBUTIONS OF GENETIC FACTORS TO NICOTINE ADDICTION
Environmental influences on substance use are typically more pronounced in adolescence than in adulthood and are associated with early onset15,16.
Part of the mechanism of damage produced by nicotine is when it acts as a gateway drug and exerts a priming effect on other drugs in the consumption sequence through global acetylation in the striatum, creating an environment prepared for the induction of gene expression and perpetuating the addictive mechanism17. For example, nicotine activates the cyclic AMP response element binding protein (CREB). This transcription factor is a switch to regulate long-term memory by modulating the expression of proteins such as FOSB and its isoform ΔFosB, being crucial in establishing addiction to most drugs18.
Evidence shows that various neurotransmitter systems influence addiction pathways, especially those encoding the nicotinic acetylcholine receptor, especially CHRNA5 and CHRNA3. Nicotine triggers the signal to release mainly dopamine which acts in one of many pathways as a positive reinforcer, causing the constant urge to smoke cigarettes. Another neurotransmitter involved is serotonin, and the SLC6A4 gene is responsible for serotonin transport and has been associated with depression and smoking19,20.
Genetic variability between populations has a significant influence on disease susceptibility and severity. Specifically, in Mexican mestizo subjects, there is an association between several genetics variants: codifying for cholinergic receptor nicotinic α4 and β2 subunits (CHRNA4, CHRNB2), DRD2, ankyrin repeat and kinase domain containing 1 protein (ANKK1), dopamine transporter (SLC6A3), and the hepatic enzyme responsible for nicotine metabolism (CYP2A6) and cigarette consumption, the age of initiation, and the duration of smoking21. In smokers, some single nucleotide polymorphisms have been identified that are associated with a reduced risk of developing chronic obstructive pulmonary disease22. It is noteworthy that the reported CYP2A6 gene variability related to tobacco use in various human populations has been identified as a primary factor regulating nicotine metabolism. This enzyme has interethnic variability and is associated with intermittent tobacco use and nicotine addiction23, which can be associated with a higher prevalence of consumption.
The evidence is suggestive that tobacco use is a partially heritable trait, more so for regular use than for onset. The expression of genetic risk for smoking among young people may be moderated by small-group and larger social-environmental factors24. According to the International Cannabis Consortium and the Tobacco and Genetics Consortium, polygenic risk scores for cigarettes per day are positively associated with lifetime e-cigarette use and early initiation of water pipe use, but only in ex-smokers (Odds Ratio [OR] = 1.4, R2=1.56%, p = 0.011) and never cigarette smokers (OR = 1.35, R2 = 1.6%, p = 0.013), respectively25.
BIOPSYCHOSOCIAL FACTORS INFLUENCING CURRENT E-CIGARETTE USE AMONG ADOLESCENTS AND ADULTS
A comprehensive understanding of risk factors potentially common to cigarette and ENDS use may help predict future cigarette and dual use trends and guide preventive interventions. Evidence supports similar psychosocial risk factors for consuming conventional tobacco products and for ENDS. In the Southern California Children's Health Study cohort, e-cigarette use, especially current use, was strongly associated with psychosocial factors shared by both e-cigarette and cigarette users. For example, 34% of current e-cigarette users had another e-cigarette user at home, compared with only 7.3% of never users (OR = 6.8; 95% confidence interval [CI]: 4.7-9.8). The presence of a cigarette smoker at home was also associated with higher odds of current e-cigarette use (OR = 2.8; 95% CI: 2.0-3.9)26. Furthermore, an association between initial e-cigarette use and subsequent cigarette smoking initiation in adolescents and young adults has been consistent. In a meta-analysis, ever e-cigarette users, and especially past 30-day e-cigarette users had increased odds for subsequent cigarette smoking (pooled OR = 3.5, 95% CI, 2.4-5.2 and OR = 4.28, 95% CI 2.5-7.3, respectively)27. In another meta-analysis among teenagers from Europe and North America, e-cigarette use was associated with the initiation of tobacco cigarette smoking (OR = 4.1, 95% confidence interval, 95%CI of 3.0–5.48)4.
Figure 2 shows the risk factors associated with the onset and development of tobacco use throughout adolescence and young adulthood.

Figure 2. Biopsychosocial factors associated with electronic cigarette and cigarette use. AOR: Adjusted Odds Ratio.
Adolescents and young adults are uniquely susceptible to social and environmental influences to use tobacco. In the Population Assessment of Tobacco and Health study cohort study representative of the non-institutionalized US population data from 3 waves between 2016 and 2019 analyzed in January 2022, found that more than 60% of adolescents reported past 30-day e-cigarette advertising exposure at each survey. Those adolescents who reported e-cigarette advertising exposure were more likely to become ever e-cigarette users (adjusted OR [aOR] = 1.21 [95% CI, 1.05-1.41]) and current e-cigarette users (aOR = 1.42 [95% CI, 1.16-1.75]) at follow-up. Also, adolescents who reported having best friends using e-cigarettes were at higher risk of current use (aOR = 5.4 [95% CI, 1.49-19.7]) than adolescents who reported having no best friends using e-cigarettes28. The evidence strongly supports the importance of peer group social influences on the initiation and maintenance of smoking behaviors during adolescence.
Negative affect and risk perception also play an important role in youth smoking behavior. Adolescents and young adults in Texas who reported depression/anxiety comorbidity reported more often the current use of e-cigarettes (aOR = 2.3; 95% CI 1.3-4.2), and current use of combustible tobacco (aOR = 3.0, 95% CI 1.3-7.1). In a meta-analysis, youths who were ever e-cigarette users perceived e-cigarettes as less addictive than tobacco cigarettes (OR = 2.28, 95% CI: 1.81-2.88)29.
ROUTES OF NICOTINE ADMINISTRATION
How nicotine is delivered to the subject plays a critical role in the risk of addiction, and it may differ depending on the device used. The product most often used to deliver nicotine is cigarettes, but other products have recently increased in popularity, such as ENDS and hookah. Some of these devices have evolved to deliver nicotine more effectively30,31.
Nicotine inhalation is the most frequently used route for achieving the desired effects in the fastest possible way. After inhalation, nicotine is rapidly absorbed into the lung thanks to the large exchange surface area with a subsequent increase in bloodstream nicotine concentrations; this route of administration allows the brain to reach high levels of nicotine in <20 s30.
Various factors that can influence the amount of nicotine administered are represented in table 2.
Table 2. Differences between nicotine delivery devices
| Characteristics | Cigarette | E-Cig | Heat-not-burn or Heated tobacco products |
|---|---|---|---|
| Components | Paper
wrapping Filter Chemical additives Tobacco |
Mouthpiece E-liquid Battery Heating coil Atomizer |
Battery Short tobacco stick Charger |
| Temperature | 900°C | 150-350°C | 350°C |
| pH | 6.5 | 7.4-9.7 | 5.8-6.7 |
| Level of nicotine | 1.99 mg/cigarette (0.2) | 69 mg/mL (0-87.2mg/mL) | 15.2 mg/g (1.1) |
| Nicotine levels per 12 puffs | 2 mg/12 puffs | 0.5-1.5mg/12 puffs | 1.45 mg/12 puffs |
| Number puff per device | 7-8 puffs | 200-400 puffs | 14 puffs |
| Flavors | > 11 variants | > 7000 | > 5 |
Puff volume, inhalation depth, rate and intensity of puffing, the particle size distribution of the aerosol, nicotine concentration, and several physical states can all modify the concentrations of nicotine delivered30,31. In addition, the absorption of nicotine through biological membranes depends on the pH; the more acidic, the lower the absorption. Not only can pH modify the absorption amount, but also protonated nicotine (nicotine salts) and nicotine lactate cause higher and more rapid absorption30,31.
Flavored additives not only increase the popularity of consumption in any device but also play an important role in nicotine dependence. Menthol, for example, has been shown to upregulate nicotinic acetylcholine receptors. Thus, there is a general understanding of the neurobiological effects of menthol plus nicotine on the brain, which include enhancing nicotine reward, altering nicotinic acetylcholine receptor number and function, and altering midbrain neuron excitability32.
Some of the problems we face in knowing the amount of nicotine contained in different devices are that some of them, especially ENDS and hookah, do not specify the exact nicotine concentration, or it is indicated in different units33.
HEALTH RISKS ASSOCIATED WITH NICOTINE
Addiction to nicotine leads globally to 14.5% of all deaths, although most of them are attributed to toxic products of tobacco combustion during smoking. E-cigarette aerosols also contain a variety of toxins, and adverse effects in vapers have been described more often. There is growing evidence that exposure of pregnant women to EC may impair placental function and may result in fetal structural abnormalities. Furthermore, it appears that using EC can cause both short- and long-term respiratory problems in the pediatric population and there are fears that a future generation of youth may be addicted to nicotine34. Nicotine by itself is not innocuous. Tobacco workers who trans-dermally absorb nicotine develop nausea, vomiting, chills, sweats, and changes in blood pressure and heart rate in what was first called "green tobacco sickness"35. NTR therapy and new studies of nicotine delivery devices have tested the effects of nicotine exposure without smoking. As shown in table 1, studies prove that nicotine poses a substantial health risk per se. Animal studies have shown that such effects do exist, although still unproven all of them in human epidemiological data. Defining better all direct nicotine health effects require further well-designed studies. Tobacco smoking is sustained by nicotine addiction and causes varied diseases and many deaths and incapacity but was not included in the table. Detailed information can be found compiled from many sources including documents from the Surgeon General of USA.
NEW CHALLENGES: DUAL AND MULTIPLE DRUG USE
In recent years, numerous investigations have been carried out to specifically identify the harm and consequences of electronic cigarettes since this is a relatively new product, and this trend becomes more complex with the implementation and ease of using these devices with other addictive substances with consequences that can be serious and devastating for public health.
Recent studies of adolescents and young adults indicate the combined use of electronic cigarettes and cannabis is more common than the use of these products by themselves36. The results of another relevant study suggest they could be the gateway to the consumption of marijuana37. In addition, they can be used to consume other drugs due to the ease of altering the odors associated with the delivered substance and the ease with which users can be concealed38. Although studies of epidemiological trends, use, frequency, and harmful consequences of these added substances in electronic devices are still in their infancy, one survey conducted in England revealed that, of 861 people who had used an electronic vaping device, more than a third (39.5%) had used it to vape other recreational drugs. The most vaped drug was cannabis (18.0% of vaping device users). However, there was evidence that users of electronic vaping devices vaped a wide variety of drugs, including MDMA (11.7%); cocaine powder (10.9%); crack cocaine (8.4%); the synthetic cathinone, mephedrone (8.5%) and α-PVP (7.1%); synthetic cannabinoids (7.8%); opioids, heroin (7.1%) fentanyl (7.3%), and other drugs, including tryptamines and ketamine39. MDMA, also known as ecstasy, is a synthetic drug with stimulant and hallucinogen effects. α-PVP, "flakka," or alpha- alpha-pyrrolidino valerophenone, is a psychoactive substance with stimulant effects, which belongs to the group of cathinones.
It is important to highlight that information exists in vaping forums, alive or in videos, on how to use other addictive substances which poses followers at risk of potentially adverse consequences like toxicity and/or addiction.
Therefore, all potential risks of using these devices must be considered since they can be the gateway to other substances, not only nicotine or cannabis. The consequences could be devastating in the prevalence of use, the age of initiation, problematic consumption, and the health effects, including addiction. The outbreak of severe acute lung injury in vapers e-cigarette-or vaping-associated lung injury (EVALI) in 2019-2020 is an example of the unexpected toxicity due to experimentation with those new devices. As of February 18, 2020, a total of 2,807 hospitalized EVALI cases or deaths had been reported to CDC from all 50 states, the District of Columbia, and two U.S. territories (Puerto Rico and U.S. Virgin Islands). Laboratory data show that vitamin E acetate, an additive in some THC-containing e-cigarette, or vaping products, is strongly linked to the EVALI outbreak, but the contribution of other chemicals of concern, including chemicals in either THC or non-THC products cannot be ruled out in some of the reported EVALI cases40.
POSSIBILITIES OF NEW TREATMENTS AGAINST ADDICTION
The first pharmacotherapeutic interventions for smoking nicotine addiction consist of NRT, the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, and the norepinephrine-dopamine reuptake inhibitor bupropion. In the area of medications, current research focused on the receptors targeted by nicotine and the brain circuits and regions known to influence nicotine consumption. Newer brain targets, including the orexin and glutamate signaling systems, have also shown promise for medication treatment41. Orexin/receptor pathways play vital regulatory roles in many physiological processes, especially feeding behavior, sleep-wake rhythm, reward and addiction, and energy balance. Orexin has been reported to modulate prefrontal and other glutamatergic inputs in dopaminergic neurons of the VTA. In addition, it highlights the recent FDA approval of the dual orexin receptor antagonist suvorexant (Belsomra®) for the treatment of insomnia as a promising sign of the potential clinical utility of orexin-based therapies for the treatment of addiction42. An alternative therapeutic strategy addresses the role of addictive drug pharmacokinetics in the addiction process. This approach uses antibodies (Abs) to prevent or slow drug entry into the brain so that the positive reinforcement from drug ingestion diminishes. A variety of preclinical anti-drug vaccines and monoclonal Abs have effectively diminished the physiological and behavioral responses associated with addiction in animals. These preclinical studies suggest that vaccines directed against misused drugs can effectively reduce addiction-related behavior43 at least temporarily.
Among the new pharmacological treatments that have shown favorable results is the amino acid cytisine, another α4β2 partial agonist. The Randomized Screening and Multiple Intervention on Lung Epidemics Lung Cancer Screening Trial44 enrolled 869 current heavy tobacco users in a low-dose computed tomography screening program, with a randomized comparison of pharmacologic intervention with cytisine plus counseling (n = 470) versus counseling alone (n= 399). At the 12-month follow-up, the quit rate was 32.1% (151 participants) in the intervention arm and 7.3% (29 participants) in the control arm. The adjusted OR of continuous abstinence, biochemically verified through carbon monoxide measurement, was 7.2 (95% confidence interval: 4.6–11.2). Additional trials of cytisine are needed to explore variations in the drug regimen and the level of behavioral support needed to boost quit rates.
CONCLUSION
Nicotine addiction, whether through smoking or vaping, impacts neurotransmitters systems, while many genetic and behavioral factors can affect its severity, the associated withdrawal symptoms, and other health risks, the most relevant from the public health point of view are those derived from tobacco smoking with huge impact in mortality and morbidity.
Emerging data show growing risks and harms associated with users of e-cigarettes and heated tobacco devices. Therefore, it is important to continue monitoring the recent research findings on nicotine addiction and the potential of e-cigarettes to impact public health. In addition, the multifactorial nature of nicotine addiction requires coordinated and multidisciplinary research.











 nova página do texto(beta)
nova página do texto(beta)


