INTRODUCTION
Psychostimulants (cocaine, amphetamines [AMPH], and cathinones) are sympathomimetic substances with effects on the central nervous system (CNS) and peripheral nervous system (PNS) similar to those produced by adrenaline and noradrenaline. Licit and illicit markets coexist for these substances, always relevant to public health. Some psychostimulants are medications for treating attention-deficit/hyperactivity disorder (ADHD), obesity, and narcolepsy, but many others are misused substances that produce severe adverse health and social effects. According to the most recent World Drug Report, the amphetamine-type psychostimulants (ATS) amphetamine (AMPH), methamphetamine (METH or "crystal meth"), 3,4-methylenedioxymethamphetamine (MDMA, "ecstasy," or "Molly"), and cathinones are the third worldwide misused drugs, preceded by cannabis and opioids. Recent data indicate that approximately 34 million people used AMPH and METH in 2020, and 20 million consumed MDMA in the same period1. This review focuses on ATS' effects and mechanisms of action.
HISTORICAL ASPECTS AND CLINICAL USE OF AMPHETAMINE-TYPE PSYCHOSTIMULANTS
ATS are a heterogenous group of natural and synthetic compounds related to AMPH. The chemist Lazăr Edeleanu was the first to synthesize AMPH in the 1880s but did not perform a pharmacological characterization (Fig. 1). Later, Nagai Nagayoshi isolated ephedrine from the Chinese herbal medicine Ephedra spp. and synthesized METH in 1893. Due to its bronchodilator and adrenaline-like actions, ephedrine was successfully introduced into the United States (U.S.) and Europe in the 1920s as a decongestant and asthma-relieving drug. In 1927, while searching for a substitute for ephedrine, Gordon Alles synthesized racemic AMPH and studied its effects in animals and humans, finding that it increased arousal and produced insomnia. Its clinical use began when Smith, Kline, and French Co. introduced AMPH into the market in 1935 under the name of Benzedrine® to treat narcolepsy, post-encephalitic parkinsonism, and depression. A few years later, the same pharmaceutical company introduced the more potent isomer dextro-AMPH (d-AMPH) marketed as Dexedrine®2. Benzedrine was freely available as a decongestant inhaler containing a cotton strip soaked in volatile AMPH oil. People soon realized that they could inhale AMPH to experience psychostimulant effects and it became extremely popular. Only in 1939 was a prescription required to buy Benzedrine and Dexedrine. At approximately the same time, Charles Bradley, an American psychiatrist, administered Benzedrine to 30 children with behavioral disorders and found a significant improvement in their school and social performance. Bradley published his findings in 1937, but this contribution remained unnoticed by the medical community for a couple of decades. However, his study became the basis for using stimulants for ADHD treatment.

Figure 1. Timeline of amphetamine-type stimulants' (ATS) most relevant events. AMPH: amphetamine, METH: methamphetamine; MDMA: methylenedioxymethamphetamine.
During World War II, soldiers used AMPH and METH pills to endure long fighting journeys, and some became dependent on these substances. Recognizing their high potential for misuse, in 1970, the U.S. Drug Enforcement Administration (DEA) and the United Nations scheduled AMPH as strictly controlled substances. Since then, most AMPHs have become illegal, except for some prescribed formulations, such as Adderall®, containing l-AMPH and d-AMPH salts for treating ADHD, narcolepsy, and obesity3.
MDMA, another relevant AMPH derivative with stimulant and hallucinogen effects, was first synthesized in 1912 by the pharmaceutical company Merck; however, it did not gain popularity until the late 1970s, particularly in the dance and rave scenes. At the same time, there were some studies on the effects of MDMA as an adjuvant to psychotherapy4, but they were inconclusive due to uncontrolled variables. By 1985, MDMA became a Schedule I drug, the most restrictive category for substances with high addiction liability and no accepted medical use. Despite this, recent studies have shown promising results for MDMA in treating post-traumatic stress disorder and anxiety5.
Other MDMA-like drugs, such as 3,4-methylenedioxyamphetamine (MDA), 3,4-methylenedioxy-N-ethylamphetamine MDEA), and 3,4-methylenedioxy-propylamphetamine (MDPA), have similar psychoactive effects and are similarly controlled due to their misuse potential.
Cathinone is an alkaloid in the leaves of the Catha edulis (khat) shrub that grows in eastern Africa and the Arabian Peninsula. It is another potent AMPH-like stimulant drug. The first cathinone derivatives, mephedrone and methcathinone, were synthesized in the late 1920s, but they appeared in the market in 2003 as part of the new psychoactive substances. Due to their recent appearance, they were unregulated, a condition used by drug sellers to advertise these cathinones as "legal." A few years later, when many countries banned mephedrone and methcathinone due to their dependence liability and adverse effects6, other derivatives, such as methylone, ethylone, and 3,4-methylenedioxypyrovalerone (MDPV) appeared for sale, usually online, advertised as "legal highs," "bath salts," "fertilizers," "air fresheners," or "chemical reagents." Their packages are labeled "not for human consumption" to circumvent regulations applicable to medications or food products. Some of these substances are more potent than cocaine and became responsible for emergency admissions of people complaining of chest pain, paranoia, hyperthermia, epistaxis, sweating, and panic attacks.
As it occurs with AMPHs, a few cathinone derivatives are medications approved by the Food and Drug Administration (FDA) in the U.S. Some examples are diethylpropion, bupropion, and pyrovalerone, prescribed as anorectic agents, for chronic fatigue treatment, as antidepressants, or for smoking cessation7.
STRUCTURAL AND PHARMACOKINETIC ASPECTS OF AMPHETAMINE-TYPE PSYCHOSTIMULANTS
ATS are sympathomimetic substances with effects and chemical structures similar to the monoamine neurotransmitters dopamine, noradrenaline, and serotonin. From the chemical point of view, ATS are phenethylamine derivatives with (a) an unsubstituted phenyl ring, (b) a two-carbon chain between the phenyl ring and the nitrogen atom, (c) an α-methyl group, and (d) the primary amino group. MDMA and its analogs contain the phenethylamine and a methylenedioxy ring, making them similar also to serotonin. On the other hand, cathinones differ from AMPHs in the presence of a ketone group (-C=O) bound to the β-carbon in the phenylethylamine (Fig. 2).
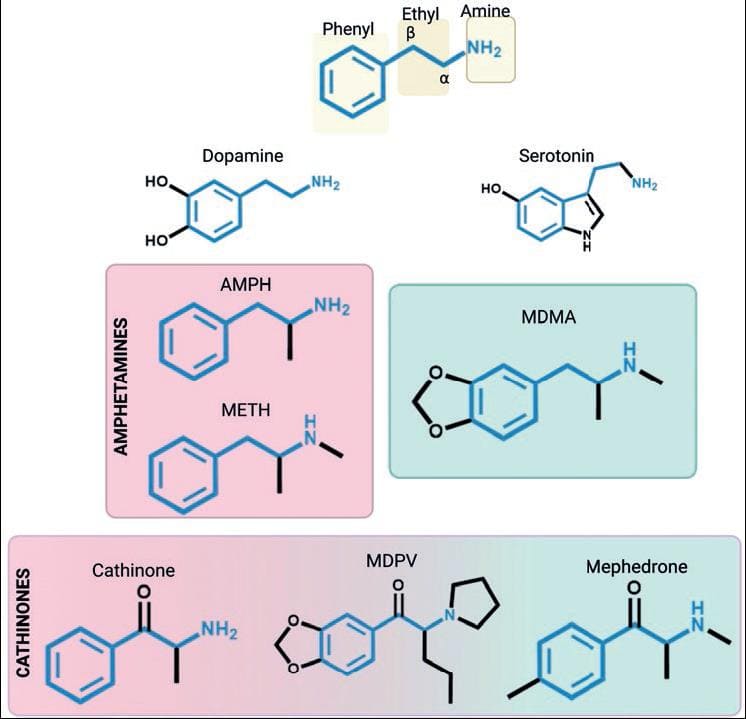
Figure 2. Chemical structures of amphetamine-type stimulants (ATS) and monoamines. The phenylethylamine core, which is shared among ATS, is highlighted. AMPH: amphetamine; METH: methamphetamine; MDMA: methylenedioxymethamphetamine; MDPV: methylenedioxypyrovalerone.
ATS exist as dextro- (d-) and levo- (l-) isomers8. The d-isomer is usually three to fourfold more potent than the l-isomer. However, the l-isomer of some cathinones can be more potent9.
ATS are available as free bases or salts, in tablets or powder, and in the case of METH, also as crystals. Depending on their presentation, they can be administered by intravenous injection, oral ingestion, smoking, inhalation, intrarectal, and intravaginal routes. Salts and powders are crystalline or white/brownish, odorless, and bitter; while ATS for medical use exist commonly in tablets or capsules as immediate-release and extended-release pharmaceutical formulations (ER or XR). Absorption, distribution, the time to reach maximal concentrations, and duration of effects vary among ATS. Table 1 summarizes the pharmacokinetic properties of the main ATS. Briefly, after non-parenteral administration, ATS are absorbed through the gastrointestinal tract and enter the bloodstream, with plasma concentrations peaking at 3-6 h. ATS are distributed through several organs, including the lungs, liver, and stomach. Due to their high solubility in lipids, they cross the blood-brain barrier and placenta and can be secreted into breast milk. Most ATS undergo liver metabolism by oxidative reactions mediated by the CYP2D6 and are excreted in the urine10-15.
Table 1. Main characteristics and chemical properties of amphetamine-type stimulants in humans
| Characteristics or properties | METH | AMPH | MDMA | Cathinone |
|---|---|---|---|---|
| Chemical name | (2S)-N-methyl-1-phenylpropan-2-amine | (2S)-1-phenylpropan-2-amine | (2S)-1-(1,3-benzodioxol-5-yl)-N-methylpropan-2-amine | (2S)-2-amino-1-phenylpropan-1-one |
| Brand names for medical use | Desoxyn [U.S.] Methampex
[U.S.] Methedrine [UK] Pervitin/Temmler [Germany] |
Adderall [U.S.] (l-AMPH +
d-AMPH) Adderall XR [U.S.] 10-25 mg (Mixture of salts) |
None | Tenuate (Diethylpropion) Wellbutrin (Bupropion) Zyban (Bupropion) |
| Street Names | Speed, meth, or chalk (referred to the salt). Crystal, crystal meth, and ice (referred to crystals | Bennies, black beauties, crank, glass, ice, speed, pep pills, uppers | Adam, ecstasy, E, eve, M&M, MDM, speed for lovers, sweeties, XTC | Bath salts, meow meow, meph, miaow, MM-cat |
| Relevant pharmacokinetic parameters | Cmax 10 mg (oral): 14-90 ng/mL Cmax 22 mg (smoked): 47 ng/mL Tmax: 2-8 h (oral), 2-3 h (smoked), 3-4 h (nasal) Protein binding: 10-20% Bioavailability: 67% (oral), 90% (smoked) Vd 3-4 l/kg |
Cmax 10 mg (oral): 15-34 ng/mL Cmax (smoked): ≈ 48 ng/mL Tmax: 2-8 h (oral), 2-5 h (smoked) Protein binding: 23-26% Bioavailability: ≈68% (oral), 90% (smoked) Vd 3-5 l/kg |
Cmax 100 mg (oral): 0.2 mg/l Tmax: 1-2 h (oral) Protein binding: 49% Bioavailability: No human data are available Vd 3-9 l/kg |
Cmax depends on the specific cathinone derivatives. Cathinone 45 mg: 59 μg/l; Cathine 32 mg: 71 μg/l; Norephedrine 18 mg: 72 μg/l Bioavailability: 45% |
| Elimination half-life time | 10-30 h | 4-15 h d-AMPH (10-11 h) <l-AMPH (12-14 h) | 6-10 h | ≈2 h |
| Main metabolites | AMPH p-hydroxymethamphetamine | AMPH sulfate p-hydroxyamphetamine noradrenaline | N-demethylation form MDA, also active, monohydroxy (HMMA, HMA), and their conjugates with glucuronide | Oxidation of keto group and N-demethylation in most cathinones |
| Urinary excretion | Most are excreted in 2-4 days. 35-45% unchanged METH, 15% p-hydroxymethamphetamine, 4-9% AMPH | Most are excreted in 2-4 days. 3-60% unchanged AMPH | 25% unchanged MDMA, 23% HMMA, 20% 3,4-diOH-methamphetamine, ~ 1% MDA | Khat (chewed): 22-52% unchanged cathinone, noradrenaline, and norpseudoephedrine |
AMPH: amphetamine, METH: methamphetamine, MDMA: methylenedioxymethamphetamine, Cmax: maximum concentration; Tmax: time to peak drug concentration; Vd: volume of distribution; MDA: methylenedioxyamphetamine, HMMA: 4-hydroxy-3-methoxymethamphetamine, HMA: 4-hydroxy-3-methoxyamphetamine, d-AMPH: dextro-AMPH; l-AMPH: levo-AMPH.
ACUTE AND CHRONIC EFFECTS OF AMPHETAMINE-TYPE PSYCHOSTIMULANTS
The behavioral and cognitive effects of ATS are related to indirect monoamine release. AMPH and METH mainly release catecholamines. MDMA and its derivatives release catecholamines and serotonin; and cathinones have a mixed mechanism of action (see below). Acute ATS administration generally increases arousal, blood pressure, and heart rate. They produce mydriasis, euphoria, and talkativeness, enhances motivation, reduces appetite, and causes insomnia (Fig. 3). High doses or chronic use of ATS cause anxiety, paranoia, cognitive deficits, and psychosis. Hyperthermia and serotonin syndrome can occur with high doses of several ATS. As with other misused substances, psychostimulant repeated use can produce addiction.
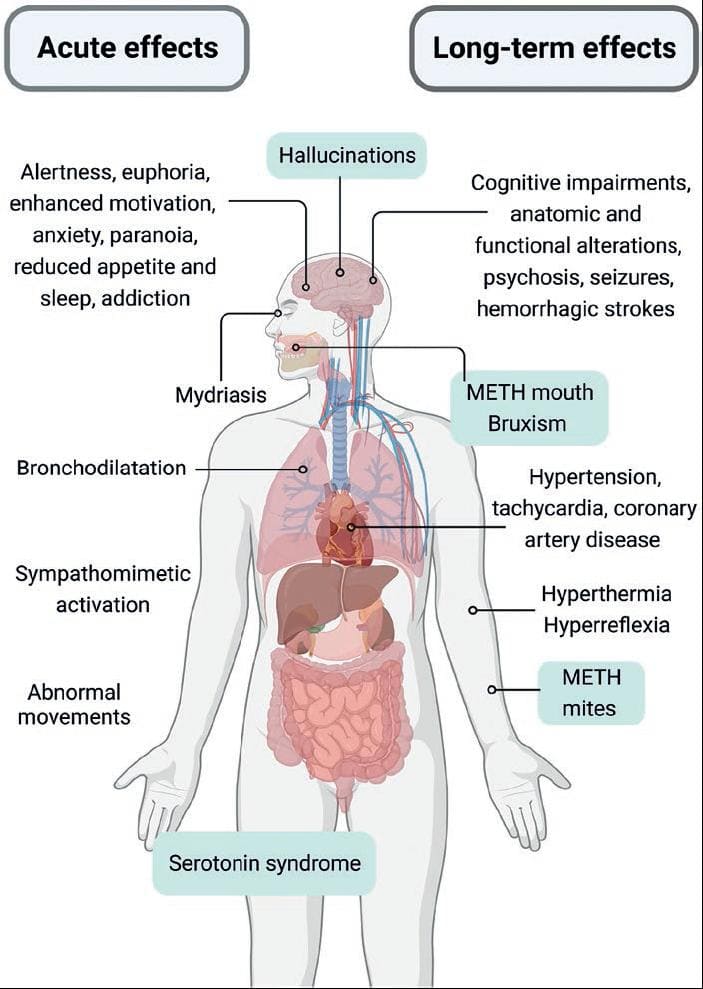
Figure 3. Acute and long-term effects of amphetamine-type stimulants (ATS). The figure summarizes the common effects for all ATS and highlights the effects related to serotonin actions after methamphetamine, MDMA, or cathinones use. Created with BioRender.com.
Cardiovascular effects
Acutely, AMPHs and cathinones have sympathomimetic actions in the cardiovascular system, increasing heart rate and blood pressure. Repeated use of AMPHs could produce hypertension, tachycardia, and coronary artery disease. All these features are related to blood vessel wall damage ranging from vascular fatigue to atherosclerosis and vessel rupture.
Other secondary effects of vascular damage are aneurysms and cerebral vasculitis, which lead to ischemia and infarction. Hypertension, cerebral vasculitis, and vasoconstriction can trigger hemorrhagic strokes (intracerebral and subarachnoid) predominantly in the frontal lobe and basal ganglia of young adult AMPHs users. Stroke occurrence (usually mini strokes) depends on the doses and time of drug use. The first signs of an AMPH-induced stroke are headache, vomiting, and confusion. In addition, transient neurological symptoms can appear (paresthesia, language problems, and visual deficiencies).
Coronary artery disease is a hallmark of chronic METH use. This disease produces intense vasoconstriction induced by noradrenaline release and α- and β-adrenergic receptor stimulation. In addition, chronic β-adrenergic receptor activation by catecholamines can lead to a transient left ventricular dysfunction in the absence of coronary artery disease called Takotsubo cardiomyopathy, resulting in weakened heart's contractile function16. METH-induced cardiotoxicity has been attributed to sympathomimetic stimulation and reactive oxygen species (ROS) production, but several mechanisms are now recognized to be involved (reviewed in Reddy et al.17).
Skin adverse effects
METH use can produce tactile hallucinations reported as bugs under the skin ("METH mites") which leads to compulsive skin picking and scratching. The neural bases of this syndrome are not well understood but may be caused by altered neural activity in the somatosensory cortex18. In addition, AMPH-induced vasoconstriction limits blood flow, which leads to tissue damage and ulcerations. Sweating and poor hygiene can exacerbate skin irritation, inflammation, and infections.
Oral adverse effects
ATS use is associated with severe oral problems. Activation of adrenaline receptors produces xerostomia, and dry mouth contributes to adverse oral effects19. Caries is the first manifestation and can progress rapidly. In addition, METH hydrochloride is acidic and causes enamel erosion. These effects are typically observed among METH users and are termed "METH mouth." It includes severe tooth decay and gum disease, resulting in missing teeth. Bruxism is also a common side effect among METH and MDMA users20. This condition is related to increased serotonin levels, jaw muscle tension, and cramps caused by electrolyte disturbances.
Cognitive impairment
The main impaired functions affected by long-term AMPH use are learning, memory, executive functions, psychomotor speed, language, and social skills. Khat-and synthetic cathinone-induced impairments are less studied, but cognitive dysfunctions are similar to those reported by AMPHs21. The relationship between cognitive impairments and ATS is difficult to determine because users usually have affective disorder comorbidities, polysubstance use, and other confounding factors22.
Seizures
METH and MDMA overdose, or binge consumption, increase the probability to develop seizures. These seizures can occur with fever, hypertension, confusion, and delirium. In addition, several factors can be involved in the proconvulsant effect of AMPHs, including an imbalance in monoamines, glutamate, and GABA levels, hyperthermia, and hyponatremia23.
Psychosis
One of the hallmarks of repeated nonmedical use of ATS is the occurrence of transient or enduring psychosis, which depends on the use pattern. Most episodic (two or more days of non-use) METH and MDMA users have had at least one psychotic episode in their lifetime. METH-associated psychosis includes distressing symptoms such as paranoid thinking, persecutory ideas, and auditory/visual hallucinations.
The symptoms emerge within hours or days of high ATS doses and usually cease with abstinence. However, METH-associated psychosis can remain for months, even after long-term abstinence24. As AMPH use escalates, psychotic symptoms become more frequent25. Recurrent drug use increases the chance of being diagnosed with a chronic psychotic disorder in the following years. Thus, the most consistent predictors of psychotic symptoms are the frequency and quantity of AMPHs used.
Addiction
ATS have a high potential for abuse and dependence due to their hedonic effects and the rapid development of tolerance, making users escalate doses and frequency of consumption. ATS users experience intense withdrawal symptoms after stopping their use. Withdrawal symptoms include headaches, tremors, chills, sweating, increased appetite, insomnia, fatigue, lethargy, depression, anxiety, and intense craving (overwhelming desire or urge to consume the drug). The severity of symptoms correlates to the level of dependence. There is a high incidence of relapses among ATS users26.
The treatment for stimulant use disorder, which includes ATS, depends on the severity of the addiction and the individual's specific needs. No approved pharmacological treatments for ATS addiction exist, but some medications can alleviate withdrawal symptoms. Behavioral therapy is the most effective treatment for AMPHs dependence.
The clinically of ATS medications for ADHD is safe, and its efficacy is well documented, providing that these substances are used by prescription, at the proper doses, and under medical supervision. However, if medical ATS are misused, that is, at higher doses, by different administration routes, or at intervals shorter than those prescribed, they can develop an addiction.
Hyperthermia
ATS increase body temperature, which could have severe consequences. The physiological basis of AMPHs-induced hyperthermia involves a combination of factors. Monoamines produce muscle hyperactivity, brown-adipose tissue activation, and decreased blood flow to the skin27. AMPH-induced hyperthermia can lead to rhabdomyolysis28, where muscle tissue breaks down and releases myoglobin into the bloodstream. Myoglobin can cause kidney damage and contribute to hyperthermia by increasing metabolic heat production. Hyperthermia, along with restricted hydration, can be fatal.
Serotonin syndrome
The concomitant use of MDMA and drugs that affect serotonin levels can produce a serotonin syndrome, characterized by confusion, restlessness, sweating, high blood pressure, muscle rigidity, headache, nausea, vomiting, diarrhea, and seizures26. The serotonin syndrome is a life-threatening condition that requires immediate medical attention. Medications that can produce a serotonin syndrome when combined with MDMA include commonly prescribed antidepressants, such as selective serotonin reuptake inhibitors, serotonin, and noradrenaline reuptake inhibitors, monoamine oxidase (MAO) inhibitors, antimigraine triptans, some opioid analgesics (fentanyl, tramadol, and dextromethorphan), and the antidepressant buspirone (Table 2)29-31.
Table 2. Adverse effects of drug-drug interactions with amphetamine-type stimulants
| Types of drugs | Effects |
|---|---|
| Drugs of abuse | |
| Cocaine | ↑ Blood pressure |
| LSD, cannabis, or mescaline | ↑ Anxiety, psychotic symptoms, serotonin syndrome, cognitive disorders |
| Opioids | ↑ Analgesic effects of opioids, ↓ sedative and respiratory depressant effects, serotonin syndrome (particularly with tramadol and fentanyl) |
| Stimulant and opioid combination is referred to "speedball" | |
| Antidepressants/anxiolytics | |
| MAO inhibitors | Hypertensive crisis, serotonin syndrome |
| SSRI or SNRI | Psychotic symptoms, serotonin syndrome |
| TCA | Hypertension, CNS stimulation |
| Other drugs | |
| Acidifying or alkalinizing agents | ↓ or ↑ Absorption and elimination of ATS |
| Anti-migraine triptans | Serotonin syndrome |
| Antihypertensive medications | ↑ Blood pressure and heart rate |
| CYP2D6 inhibitors | Potential pharmacokinetic interactions with cocaine, ATS, or new psychoactive opioids |
| Protease inhibitors | ↑ MDMA or METH concentration, leading to drug toxicity, and potential death |
| Sodium oxybate | Potential unconsciousness |
| St John's wort | Serotonin syndrome |
ATS: amphetamine-type stimulants; CNS: central nervous system; LSD: lysergic acid diethylamide; MAO: monoamine oxidase; METH: methamphetamine; MDMA: N-methyl-3,4-methylenedioxymethamphetamine; SNRI: serotonin-noradrenaline reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; TCA: tricyclic antidepressants.
MECHANISMS OF ACTION
ATS act as indirect monoamine agonists by releasing dopamine, serotonin, noradrenaline, and adrenaline (Fig. 4A). AMPHs enter the synaptic terminal of monoamine neurons in two ways, as substrates of dopamine, serotonin, and noradrenaline transporters (NAT) Dopamine transporter [DAT], Serotonin transporter [SERT], and by passive diffusion32. Once inside the neurons, they bind to vesicular monoamine transporter type 2 (VMAT2) and enter the neurotransmitter vesicles. Because ATS are weak bases (pKa values range from 8.7 for MDMA to approximately 10 for METH), they alter the pH of the intravesicular medium and facilitate the release of monoamines into the cytosol by reversing the direction of VMAT2. Once in the cytosol, monoamines bind to DAT, NAT, or SERT and are released into the synaptic cleft through the reverse function of their transporters33.
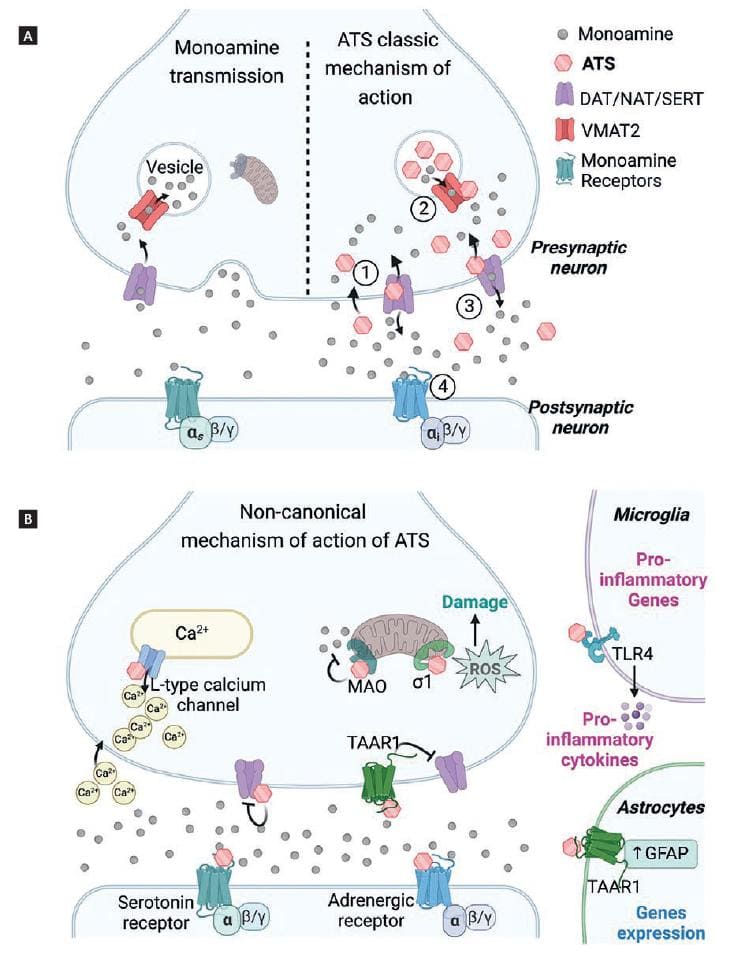
Figure 4. Mechanism of action of amphetamine-type stimulants (ATS). (A) Representation of the classic mechanism of action of ATS in monoaminergic neurons. In comparison to monoamine physiological transmission (left), ATS produced excessively high monoamine concentrations in the presynaptic terminal and the synaptic cleft (right). First, ATS enter the presynaptic terminal 1. In the vesicles, ATS release monoamines to the cytoplasm 2. Non-vesiculated monoamines are released to the synaptic cleft by the reversal transport 3. Finally, monoamines activate their monoaminergic receptors 4. (B) Non-canonical mechanisms of action of ATS. Created with BioRender.com.
Most cathinones are DAT, NAT, or SERT substrates, but others directly block monoamine transporters, producing their accumulation in the synaptic cleft. In addition, some cathinones have a mixed mechanism of action (Table 3)34-36.
Table 3. Cathinone's classification and psychological effects
| Category | Examples of compounds | Affinity to reuptake transporter | Main psychological effects |
|---|---|---|---|
| Selective DAT Substrates. "AMPH-like profiles" | Cathinone Methcathinone Flephedrone 4-MEC |
NAT ≥ DAT >> SERT | Alertness and arousal, euphoria, wakefulness, and enhanced motivation |
| Substrates for all monoamine uptake transporters. "MDMA-like profiles" | Methylone Benzedrone Mephedrone Butylone Ethylone |
SERT > NAT >> DAT | Prosocial effect, altered perception, decreased sleep and appetite |
| Monoamine uptake transporter blockers "Mixed amphetamine and MDMA-like profiles" | MDPV α-PVP α-PVT MePPP Naphyrone 1-Naphyrone |
NAT ≈ DAT ≈ SERT | Same as the previous cathinones. Euphoria, delusions, paranoia, and hallucinations |
AMPH: amphetamine; DAT: dopamine transporter; α-PVP: α-pyrrolidinopentiophenone; α-PVT: α-pyrrolidinopentiothiophenone; MePPP: 4-methyl- α -pyrrolidinopropiophenone; MDMA: 3,4-methylenedioxymethamphetamine; MDPV: methylenedioxypyrovalerone; NAT: noradrenaline transporter; SERT: serotonin transporter; 4-MEC: 4-methylethcathinone.
A significant difference among ATS is their distinct DAT and NAT versus SERT affinities. AMPH, METH, cathinone, methcathinone, mephedrone, and 4-methylethcathinone have more affinity for DAT than SERT, that is, they release more dopamine than serotonin, causing wakefulness, euphoria, arousal, and increased attention. Conversely, MDMA, and other cathinones such as methylone, benzedrone, mephedrone, and butylone have more affinity for SERT than DAT and predominantly release serotonin37. These substances increase emotions and empathy and produce hallucinations.
In addition to the above-mentioned classic mechanisms, some ATS partially or fully activate serotonin and adrenergic receptors directly (Fig. 4B). For example, MDMA, mephedrone, flephedrone, and methcathinone act directly on the 5-HT2A receptor, producing hallucinogen and psychotic effects38. Furthermore, recent studies show that ATS can also activate the trace amine-associated receptor 1 (TAAR1), a G-protein-coupled receptor that negatively modulates the dopaminergic system and down-regulates DAT expression. Interestingly, there is evidence that TAAR1 activation by a selective agonist reduces the reinforcing properties of psychostimulants39,40. These findings suggest that TAAR1 could be a potential therapeutic target to reduce the dopaminergic (and rewarding) effects of some ATS. Cathinones interact less with TAAR1 than AMPHs, which means that DAT remains on the cell surface contributing to monoamine release and increasing the risk of dependence39.
Chronic ATS and binge administration (use of continuous high doses of psychostimulants over hours or days) produce long-lasting adaptive changes and damage to central dopaminergic and serotonergic neurons, particularly in the frontostriatal pathways. In particular, some chronic METH users show reduced dopamine markers associated with poor psychomotor and cognitive performances41.
The neurotoxic mechanisms of AMPHs and cathinones are not fully understood but involve excitotoxicity caused by excessive glutamate release, blood-brain barrier damage, mitochondrial dysfunction, neuroinflammation, DNA damage, and oxidative stress. These damages are not permanent, and users can recover, at least partially, by protracted abstinence42.
METH directly binds to the sigma-1 receptor, leading to mitochondrial dysfunction and increased ROS production43. In addition, AMPH and METH inhibit MAO, the mitochondrial enzyme responsible for monoamine degradation44. Furthermore, METH increases intracellular calcium levels in two ways, by stimulating the release of intracellular stores through L-type calcium channels, and by promoting extracellular calcium entry. Excessive calcium initiates oxidative stress pathways45.
ATS produce other effects through non-canonical mechanisms. For example, high METH doses produce microglial activation by directly binding to Toll-Like receptors 4 (TLR4)46, producing neuroinflammation, and, eventually, damage to monoaminergic neurons.
EFFECTS OF ATS ON THE IMMUNE SYSTEM
Preclinical studies have shown that ATS produce significant immune cell dysfunction in the CNS and PNS, which might correlate with a higher risk of infections and other adverse effects in humans. The following sections summarize some relevant findings on this topic.
Effects on microglia and astrocytes
Microglia and astrocytes regulate homeostasis in the CNS. Microglia are resident macrophages specialized in sensing signs of injury and infection. In addition, these cells play essential roles in immune defense, synaptic pruning, and neurodevelopmental processes.
As mentioned, microglia cells express TLR4, membrane receptors that recognize pathogen-associated molecular patterns and damage-associated molecular patterns.
Recent studies have shown that METH activates microglia by binding to the same site of the TLR-4 accessory protein myeloid differentiation protein 2 where lipopolysaccharide (LPS, a membrane component of Gram-negative bacteria) binds. This increases pro-inflammatory cytokine levels, which could contribute to the neuroinflammation and neurotoxicity processes observed after repeated METH use47. Moreover, METH induces apoptosis in human and mouse microglial cell lines. Interestingly, TLR4 antagonism prevents the behavioral and toxic effects of AMPHs, suggesting that TLR4 could be a promising therapeutic target for treating METH users48,49. In addition, the finding that TLR4 antagonists can suppress dopamine release and some drug-seeking behaviors reinforces this possibility50.
Astrocytes are the most abundant cells in the CNS. They provide metabolic and structural support to neurons in the CNS, maintain the integrity of the blood-brain barrier, regulate ion and neurotransmitter levels, provide energy substrates to neurons, and repair processes after injury51. Repeated METH use produces astrogliosis, a defensive reaction characterized by structural and functional changes in response to CNS damage. In particular, astrocyte activation has been observed in the striatum, prefrontal cortex, and hippocampus of chronic METH users. It was initially proposed that astrogliosis resulted from damage to dopaminergic neurons; however, preclinical evidence has shown that METH directly activates astrocytes even after depleting dopamine, and this effect has been related to METH-induced ROS production52.
A well-described function of astrocytes is regulating extrasynaptic glutamate levels. METH also affects astrocyte-mediated glutamatergic uptake, which increases excitability during withdrawal. Furthermore, repeated METH use reduces the number of contacts between astrocytes and presynaptic neurons53. Although these alterations have been proposed as underlying relapse in ATS users, further research is needed to understand the mechanisms involved in astrocyte-mediated glutamate dysfunction.
Effects on peripheral immune cells
ATS can weaken innate and adaptive immune responses, affecting various immune cells and causing immunosuppression. Moreover, ATS use can exacerbate or worsen the effects of various transmissible diseases, including HIV, hepatitis, and tuberculosis. This section provides information on the adverse effects of METH, the best-studied stimulant, on the peripheral immune system.
METH negatively affects leukocyte migration, phagocytosis, and the killing of pathogens. METH alters macrophage function and signaling, increases ROS production, and releases matrix metalloproteinase-9. This latter effect causes damage to the blood-brain barrier, facilitating the infiltration of macrophages and neutrophils into the CNS in mice54. Furthermore, METH increases the pH level of acidic organelles inside macrophages, diminishing their phagocytic capacity and antigen presentation processes55.
METH increases the risk of acquiring infectious diseases, including hepatitis C, cutaneous bacterial infections, and sexually transmitted diseases56. In mice, repeated METH administration reduces the number of dendritic cells. METH also affects the macrophages' function impairing the innate antiviral mechanisms and contributing to the spread of HIV infection57,58.
METH can decrease human neutrophil function59. In rodents, METH facilitates neutrophil infiltration in various organs, leading to necrosis, atrophy, and other damages60. METH also impairs mast cells' activation, TLR4 expression, and cytokine production in LPS-treated mice through dopamine receptor activation in the bone marrow and intestine61,62.
METH causes tissue injury, induces apoptosis, and affects the proliferation and recruitment of cytotoxic and helper T-cells in mice challenged with LPS63. METH also decreases CD3, CD28, and IL-2 expression in human T-cells, suppressing T lymphocyte activation, and proliferation. There are reports of excessive systemic inflammation, chronic B-cell activation, and high IgG3 production in users who inject ATS64. In addition, METH alters IgM (the first antibodies produced during an immune response) levels in rodents65. These data support that METH use makes individuals more vulnerable to diseases and infections.
CHALLENGES POSED TO USERS AND HEALTH PRACTITIONERS BY RECENT TRENDS IN METH USE
METH, in its crystal form, is highly pure66, but as powder or pills, it can be adulterated with harmful substances that can cause serious health effects. Adulteration refers to adding inert or pharmacologically active substances to misused substances to increase their volume or psychoactive effects. Caffeine, ephedrine, levamisole, lidocaine, and ketamine are common AMPH and METH adulterants67. Cathinones and MDMA can be mixed with other cathinones (e.g., methylone, mephedrone, butylone, MDPV, and α-PVP, also known as "Flakka"), other misused substances (AMPH, ketamine, and phencyclidine) or new psychoactive substances68,69. These combinations increase the risk of serious cardiovascular and neurological complications.
Of particular concern is the addition of highly potent synthetic opioids, such as fentanyl and its analogs, to ATS66,67. Before 2016, overdose cases due to METH and opioid combinations were almost nonexistent. In contrast, the deaths produced by METH and synthetic opioids in the U.S. in 2020 and 2021 exceeded those caused by METH alone70. Furthermore, in Canada, a high proportion (53%) of fatal opioid overdoses involved a psychostimulant substance71.
Intentional or unintended polydrug use can potentiate individual harmful effects and produce drug-drug interactions with misused substances and medications, making the stabilizing of agitated patients or counteracting life-threatening overdoses difficult (Table 2). Users who intentionally consume opioids and METH want to experience the desired subjective effects of both substances, avoid excessive sedation, and have milder withdrawal symptoms72. Unfortunately, studies on drug adulteration are scarce, making it challenging to prevent potentially fatal overdoses and health complications.
In Mexico, the last National Household Survey was conducted in 2016, showing a lifetime prevalence of METH use in the general population of only 0.9%73. However, the use of METH has significantly increased in the past years, becoming the second substance of choice to initiate drug use74. Furthermore, METH use is the leading cause for seeking treatment in government and non-governmental institutions. For example, in 2017, METH users represented 14.5% of people attending treatment centers, but this figure increased to approximately 50% in 202275,76.
A recent study conducted in a northern city of Mexico bordering the U.S. found METH mixed with fentanyl in drug residues77. Another study reported that counterfeit "Adderall" pills are sold without prescription in northern Mexican cities, containing METH instead of the original formulation78. The availability and affordability of METH, alone or adulterated with fentanyl, require immediate attention. Prevention campaigns, drug-testing services, and raising awareness among users and health providers are necessary to prevent a sanitary crisis. In addition, it is critical to provide easy access to naloxone, the opioid antidote, to treat METH users in case of accidental opioid overdose.
CONCLUSIONS
Research on ATS has led to several novel insights into pharmacological properties and a comprehensive description of the ATS effects on the CNS and immune systems. This knowledge can guide prevention efforts and the development of effective treatments for ATS addiction and related health issues. However, increased polydrug and opioid use with ATS may favor unpredictable physiological adverse effects and accidental overdoses. This review highlights the need for evidence-based practices to reduce the potential damage of METH use, alone or combined with opioids.











 nueva página del texto (beta)
nueva página del texto (beta)


