INTRODUCTION
Prostate cancer (PCa) is one of the most common non-cutaneous malignancies among men1. The broad clinical spectrum of PCa goes from indolent forms of the disease to potentially lethal cancer. Due to this clinical heterogeneity, overdiagnosis and overtreatment have become dubious issues1,2. At present, the clinical relevance in the screening and detection of PCa is focused on the detection of clinically significant PCa (CSPCa). Defined as a histopathological Gleason score > 3 + 4 according to the International Society of Urologic Pathology (ISUP) Grade 2 or higher, this grade group classification is based on the sum of the most predominant Gleason pattern (graded from 3 to 5 depending on distinct morphological patterns from normal cells to tumor cells) and a second Gleason grade to the second most predominant pattern in the biopsy sample, ISUP grading from 1 (Gleason score 6) to 5 (Gleason score 9-10)3.
The advances in imaging technology have led to improvements in early detection, whereby the detection of CSPCa has become more frequent for urologists4,5. Prostate magnetic resonance imaging (MRI) has been in use for nearly two decades. Initially, its utility was mainly for disease localization and staging after histopathologic diagnosis was reached4. At present, it is used not only for detecting suspicious foci but also for targeting the prostate biopsies5. Three techniques of MRI-guided biopsy are available: (1) In-bore MRI targeted biopsy; (2) MRI-transrectal ultrasound fusion; and (3) Cognitive fusion MRI biopsy. No significant differences were found in the detection rates (DRs) of PCa between the three techniques; however, the cognitive fusion MRI biopsy is the most economical alternative compared to the others5.
Considering that the tumor DR drops to 18% in the second systematic biopsy6, multiparametric MRI (mpMRI) improves diagnostic performance through a better selection of patients with suspicious lesions before the biopsy using prostate imaging reporting and data system (PIRADS). PIRADS is a structured reporting scheme for mpMRI in the evaluation of suspected PCa that predicts the probability of a CSPCa, graded from 1 (unlikely to be present) to 5 (highly likely to be present)5. It is a particularly useful tool in patients with high prostate-specific antigen (PSA) and previous negative biopsies, already documented in earlier prospective studies7,8.
Despite newly standardized protocols for the interpretation of lesions suggestive of PCa, taking into account variability in experience and precision of the radiologists, it is not yet clear whether it is valid to ignore systematic prostate biopsies in patients undergoing repetitive prostate biopsies9. We studied the rate of CSPCa detection of systematic and targeted cognitive biopsies in a Mexican cohort with the previous negative systematic biopsies. A secondary objective was to describe the value of PSA density (PSAd) and prostate volume in the detection of CSPCa.
MATERIAL AND METHODS
A prospective, single-center, and longitudinal analysis was conducted from 2016 to 2021 at the National Institute of Medical Sciences and Nutrition in Mexico, comparing the DR of two types of prostate biopsies: systematic and cognitive fusion MRI biopsy. All relevant clinical data were obtained from the medical charts and corroborated with the patient if needed: age, number of previous negative prostate biopsies, prostate MRI volume, rectal examination, number, and grade of PIRADS lesions. Laboratory data included: PSA (total PSA in a blood sample expressed in ng/mL) and PSAd (total PSA (ng/mL) divided by prostate volume (mL) expressed in ng/mL2). During the biopsy, the following parameters were collected: number of fragments per region of interest (ROI), and laterality of ROI. After biopsy, histopathological data included PCa detection graded by ISUP in systematic and in cognitive fusion fragments.
Patients with a history of systematic prostate biopsy for PCa detection and that underwent prostate MRI with a 1.5 Tesla equipment were included. The images were analyzed and evaluated by two radiologists, in two different time periods, based on high-resolution sequences with T1 weighted images, T2, dynamic sequences after contrast administration, and ADC diffusion-map sequences. Subsequently, the radiologists assigned the degree of suspicion of the ROI, and this was classified according to the PIRADS V2.0 system (Grade from 1 to 5)10. We excluded patients under active surveillance. Patients with PIRADS lesions > 3 underwent a cognitive fusion transrectal ultrasound-guided biopsy in coordination with a radiologist, taking 2-4 fragments per ROI, followed by a systematic biopsy of 12 fragments. Previous to the procedure, prophylaxis with amikacin 1 g IV was administered. The histopathological analysis was made by a single pathologist.
For statistical analysis, simple descriptive statistics, such as frequencies and means, were used to find significant differences between groups. We used Student’s t-test or Mann–Whitney U-test for continuous variables and Chi-square or Fisher Exact for categorical variables. To analyze clinical agreement, Cohen’s weighted Kappa index was used. Statistical significance was predetermined to be present for values of p < 0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 25.0 (SPSS Inc, Chicago, IL).
This study was performed in accordance with the Declaration of Helsinki and the principles of good clinical practice. All patients provided written informed consent before the procedure, and the study was previously approved by the institutional scientific and bioethics committees.
RESULTS
Patient characteristics are shown in table 1. A total of 111 patients with PIRADS lesions > 3 were included; the total number of lesions were 198, and the distributions of PIRADS 3, 4, and 5 were 64 (32%), 103 (52%), and 31 (16%), respectively. The median of previous negative biopsies was 2 (IQR 1-3), with PSAd > 0.15 in 40.5%. Of the overall lesions, 42% (83/198) were located in the peripheral zone. The overall detection of PCa was 41.4% (46 of 111 patients), of which 42 (91.3%) were detected by systematic biopsy and 30 (65.2%) by targeted biopsy. Only 13.3% (4/30) positive results of the cognitive fusion MRI biopsies were not detected by systematic biopsy, whereas 38% (16/42) positive results of the systematic biopsy were not detected by targeted biopsy. Only six cases of ISUP 1 were detected by targeted biopsy, while 14 were found in the systematic biopsy.
Table 1. Characteristics of patients undergoing biopsy for prostate cancer diagnosis
| Characteristic | No. (%) n = 111 |
|---|---|
| Age at biopsy, years; mean (SD) | 66.27 (6.85) |
| Clinical T stage | |
| T1c | 79 (71) |
| T2a | 23 (21) |
| T2b | 9 (8) |
| Prostate specific antigen, median (IQR), ng/mL | 9.9 (1.21-26) |
| Prostate volume by magnetic resonance imaging, cm3 | |
| < 30 | 7 (6.3) |
| 30-60 | 37 (33.3) |
| 60-100 | 45 (40.6) |
| > 100 | 22 (19.8) |
| PSA density, ng/mL2 | |
| < 0.15 | 66 (59.5) |
| > 0.15 | 45 (40.5) |
SD: standard deviation; IQR: interquartile range.
The overall detection of CSPCa was 23.4% (26/111), of which 88.5% (23/26) were found on the systematic biopsy and 76.9% (21/26) by targeted biopsy, statistically significant (p = 0.0001) when compared with all the patients included in the study (n = 111) (Fig. 1). PSAd > 0.15 was directly associated with detection of CSPCa in 84.6% (22/26; p = 0.0001) (Fig. 2). Meanwhile, a prostate volume < 60cc was associated with the detection of CSPCa (p = 0.002).
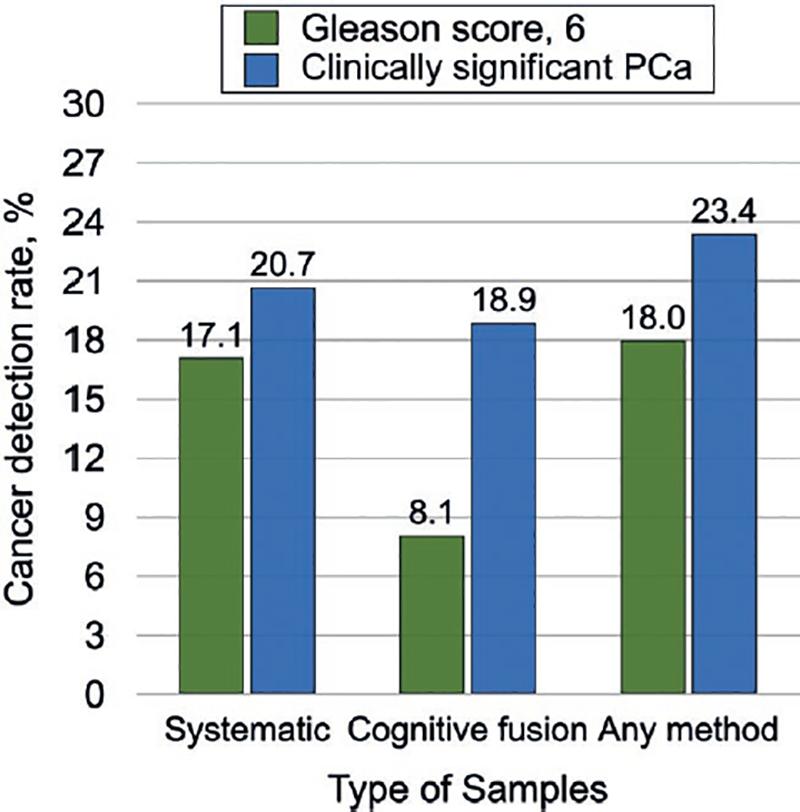
Figure 1. Cancer detection rate of Gleason score 6 prostate cancer and clinically significant prostate cancer by systematic, targeted, or any biopsy.
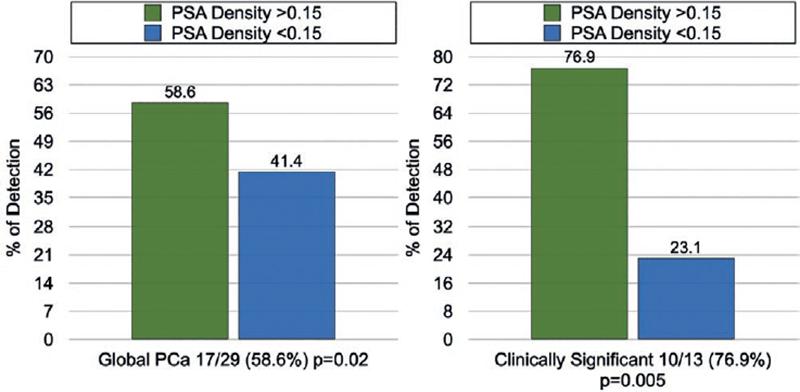
Figure 2. Rate of detection of global and clinically significant PCa in relation to PSA density > 0.15 or < 0.15.
Cancer detection based on tumor locations between targeted and systematic biopsy is shown in table 2, with an agreement of 81.3%, a Cohen’s weighted Kappa index of 0.56, moderate agreement. When only CSPCa was considered, there was a 92.3% agreement, a Cohen’s weighted Kappa index of 0.70, substantial agreement. The distribution by PIRADS 3, 4, and 5 and their relationship with overall and CSPCa is shown in figure 3.
Table 2. Location of cancers detected by targeted versus systematic biopsy (n = 111)
| Location of cancer on systematic biopsy | Location of cancer on targeted biopsy, No. (%) | ||||
|---|---|---|---|---|---|
| Negative | Right | Left | Bilateral | Total | |
| Negative | 65 (58.6) | 3 (2.7) | 1 (0.9) | 0 (0) | 69 |
| Right | 10 (9) | 6 (5.4) | 0 (0) | 0 (0) | 16 |
| Left | 5 (4.5) | 0 (0) | 13 (11.7) | 1 (0.9) | 19 |
| Bilateral | 1 (0.9) | 3 (2.7) | 0 (0) | 3 (2.7) | 7 |
| Total | 81 | 12 | 14 | 4 | 111 |
DISCUSSION
With the introduction of cognitive prostate biopsies, the operator creates a mental three-dimensional representation of the prostate and of the lesion within it, even if it is not visible on ultrasound. It is a quick method and requires no additional hardware11,12. To this day, international guidelines advise performing MRI before a repeat biopsy in patients with prior negative biopsy and suspicion of PCa3. It was recently reported that in biopsy-naïve patients, targeted biopsy detected more CSPCa than systematic biopsy. However, they showed that the yield was highest when the two approaches were combined13,14. mpMRI has a high correlation with radical prostatectomy in the detection of CSPCa, with a pooled sensitivity of 0.91 (95% CI: 0.83-0.95) and a pooled specificity of 0.37 (95% CI: 0.29-0.46)15. Kasivisvanathan et al., in a systematic review and meta-analysis comparing the DR of targeted biopsy versus systematic biopsy, found that targeted biopsy detected more men with CSPCa than systematic biopsy (DR 1.16 [95% CI: 1.09-1.24], p < 0.0001), and fewer patients with clinically insignificant cancer (DR 0.66 [95% CI 0.57–0.76], p < 0.0001)16. A relevant characteristic was avoiding overdiagnosis of indolent cancer. A negative predictive value (NPV) varied depending on study design, cancer prevalence, and definitions of positive mpMRI and CSPCa17. Consequently, it is not limited to a tool before the biopsy procedure, but optimizes the patient selection process18,19.
This study shows how the combined use of both types of biopsy increases the detection of PCa in patients with the previous negative biopsies (23.4%), considering that the detection of PCa is around 30% and decreases to 18%, 16%, 12%, and 7% in the following 2-5 subsequent procedures20. The DR of clinically significant cancer was 40.8%, higher than that obtained in a conventional way, which could indirectly reflect the usefulness of MRI in the selection of these patients21.
Previously, the utility of PSAd in the detection of PCa in guided biopsies was reported. The combination of this feature with clinical parameters and mpMRI could provide a measurable benefit in making decisions to perform biopsies in men at risk of PCa and reduce the proportion of men with undetected cancer with a threshold of 0.1522,23. The additional inclusion of clinical parameters to mpMRI could provide an added benefit in making the decision to biopsy men at risk, maintaining a high rate of CSPCa diagnosis24,25. Based on a meta-analysis, omitting the systematic biopsy would result in missing 19% of all PCa cases, and 10% of CSPCa cases. Simultaneously, by omitting the systematic biopsy, 50% of the indolent PCa would not be detected and would thereby decrease overdiagnosis of these tumors11,26. Recently, the trio study demonstrated that the addition of MRI-targeted biopsy to systematic biopsy led to 9.9% more PCa diagnoses. In that study, they used as a reference radical prostatectomy for verifying biopsy results and confirmed the superiority of combined prostate biopsy27. Remarkably, in our study, almost 15% of CSPCa would have not been detected if a systematic biopsy was omitted.
Bryk et al., suggest the use of systematic biopsies added to fusion biopsies only on the side of MRI ROI. In our study, there was a percentage of agreement of 92.3% and a Cohen’s weighted Kappa index of 0.70, which represents a substantial agreement in laterality correlation for CSPCa between cognitive and systematic biopsies, leaving opened the option to perform only systematic biopsies on the side of the MRI ROI, as a less invasive alternative28.
The limitations of our study include performance bias in systematic biopsy, since it is not possible to ignore the insertion point of a prior targeted biopsy. Furthermore, considering that biopsies were performed by different urologists, variability of the procedure experience was not measured in this study. Finally, mpMRI was evaluated by two radiologists independently, not being able to evaluate the interobserver variability, a disadvantage of any study involving the use of this imaging method, as has been reported in other studies previously9,29.
In summary, mpMRI potentially increases the performance of PCa diagnostics in combination with routine biopsies and PSAd. The detection of PCa by systematic biopsies in this series was higher than 80%, a reason why their routine use is not replaced by targeted biopsies and continue to be the cornerstone of the diagnosis of this disease, leaving open the option to perform only systematic biopsies on the side of the MRI ROI.











 text new page (beta)
text new page (beta)



