INTRODUCTION
Lymphomas comprise a heterogeneous group of hematological malignancies classified according to their clinical and histopathological features and their cytogenetic markers1. Diffuse large B-cell lymphoma (DLBCL) is the most common of all aggressive lymphomas2. Initially, clinical scores, including the International Prognostic Index (IPI) score and the revised-IPI score, provided predictive information for early relapse or progression and aided in developing stratifying tools3-5. However, these scores were developed before the introduction of rituximab and, therefore, may not be fully applicable to the current therapies, which incorporate this monoclonal antibody, as R-CHOP.
DLBCL has been subdivided into morphological variants, molecular subtypes, and distinct disease entities. The germinal center (GC-B) subtype has a significantly better prognosis than the non-germinal center (non-GC) subtype. The overexpression of MYC (> 40%)/BCL2 (>50%) proteins in the absence of cytogenetic abnormalities is known as double protein expression lymphoma and has a more aggressive clinical behavior with poor response to standard treatments6. The characterization of active pathways in the non-GC subtype, such as nuclear factor-kappa B (NF-κB) or Bruton's tyrosine kinase (BTK), suggested that the addition of targeted therapies such as lenalidomide and ibrutinib, respectively, to the standard R-CHOP regimen could improve the results, which was observed in Phase II studies7,8. However, randomized Phase III trials failed to confirm any benefit9.
Epigenetic modifications play critical biological roles because they regulate gene expression by modifying chromatin organization and regulate transcription initiation, elongation, splicing, and termination. Polycomb repressive complexes 1 and 2 (PRC1, PCR2) play a significant role in normal hematopoiesis by promoting pluripotency maintenance and self-renewal of adult stem cells. During lymphopoiesis, EZH2 is strongly expressed in proliferating cells, such as human GCB cells10. High EZH2 expression in lymphomas correlates with increased proliferation, tumor cell aggressiveness, and poor prognosis11. Mutations at Y641 in the SET catalytic domain of EZH2 in patients with DLBCL and follicular lymphoma12 correlate with poor prognosis. Since the mutation frequency varies among populations and primary site of lymphoma13-15, the objective of this study was, first, to analyze the mutational frequency of EZH2 and then its prognostic role in a cohort of Mexican-Mestizo patients with DLBCL, in terms of clinical responses, relapses, PFS, and OS.
METHODS
Cohorts
This is a cohort study of consecutive patients diagnosed with DLBCL who attended the Lymphoma Clinic at the National Cancer Institute (Mexico City, Mexico) between January 2015 and December 2017. Candidates were invited to participate if they met the following inclusion criteria: older than 18 years, a histopathological diagnosis of DLBCL, previously untreated and candidates to receive R-CHOP, and with an adequate sample for EZH2 mutation status analysis. Cohorts were defined according to the status of EZH2 mutations. Patients receiving any other treatment regimen and/or those with an active infectious disease such as hepatitis B, hepatitis C, or human immunodeficiency virus (HIV) were excluded from the study.
The study protocol was composed in compliance with the STROBE recommendations and was reviewed, then approved by the IRB (register number CEI/966/15). All patients signed the informed consent form.
The clinical variables were collected prospectively after the patients accepted to participate, and included age and gender, basal blood hemoglobin, basal serum albumin, total lymphocyte count, prognostic nutritional index (PNI) score, lactate dehydrogenase (LDH) levels, β2-microglobulin levels, IPI score, presence of B symptoms, bulky disease, Lugano staging classification16, and Eastern Cooperative Oncology Group (ECOG) performance status score17.
Histopathologic variables were GCB subtype versus non-GCB subtype, which was determined by the Hans nomogram18, as well as BCL2, BCL6, and MYC expression and double-hit lymphoma.
Immunohistochemistry (IHC) protocols
Immunostaining was performed with an automated immunostainer (BenchMark ULTRA) using the following antibodies: CD20 (Dako, clone L26 1:400), CD3 (Dako, Polyclonal 1:200), CD10 (Cell Marque, clone 58C8 1:50), BCL2 (Cell Marque clone 124 1:100), BCL6 (Cell Marque, clone G191E/AB 1:200), MUM1 (Dako, clone MUM1p 1:200), and MYC (Cell Marque, clone EP1321 1:150).
Fluorescence in situ hybridization (FISH) protocols
To identify BCL2, BCL6, and MYC rearrangements, FISH studies were performed. All of the specimens were examined to identify tumor cell enriched areas. The commercially available Break Apart probe kits specific for BCL2 (Vysis; Abbot Molecular, Abbot Park, IL), BCL6 (Vysis; Abbot Molecular, Abbot Park, IL), and MYC (CGI) were used according to the manufacturers' protocols. Results were analyzed using a fluorescence microscope (Zeiss, AXIO-A1) under a ×100 objective lens with oil immersion.
Chemotherapy regimens, follow-up, and outcomes
All patients were treated with 6 cycles of the R-CHOP regimen: IV rituximab, 375 mg/m2 on day 1; IV cyclophosphamide, 750 mg/m2 on day 1; IV doxorubicin, 50 mg/m2 on day 1; IV vincristine, 1.4 mg/m2, with capping at 2 mg, on day 1; and oral prednisone, 100 mg daily on days 1-5.
During the 1st year, all patients had outpatient clinical follow-up at the lymphoma clinic with clinical history, physical examination, blood cytology, and clinical chemistry every 3 months and computed tomography (CT) every 6 months; then, the follow-up was every 6 months with a clinical evaluation, laboratory parameters, and CT scan.
The analyzed outcomes were CR after 6 cycles of R-CHOP by positron emission tomography-computed tomography (PET-CT), using Deauville criteria19, frequency or relapses (defined initially by CT scan, and confirmed by PET-CT), PFS (measured on the diagnosis date until relapse or last visit), and OS (assessed on the diagnosis date until death or last visit). The last follow-up for all active patients was on November 25, 2020.
Samples
DNA was extracted from paraffin-embedded tissue samples using the All Prep® DNA/RNA FFPE Kit (50) (Qiagen Cat. No. 80234). PCR experiments were performed in a total volume of 25 µL that contained 50 ng of template DNA, 10 mmol/L Tris-HCl (pH 8.3), 40 mmol/L KCl, 2 mmol/L MgCl2, 200 mmol/L each dNTP, 1 U Platinum Taq DNA Polymerase (Applied Biosystems), and 1 mmol/L each specific primer (forward: 5'-ATCTATTGCTGGCACCATCT-3' and reverse: 5'- CCAATCAAACCCACAGACTTAC-3'). An initial denaturation at 94°C for 5 min was followed by 45 cycles of amplification and a final extension step for 7 min at 72°C. The amplification cycles included denaturation at 94°C for 30 s, 30 s of annealing at 58°C, and 30 s of amplification at 72°C. PCR experiments were carried out in a 2700 Thermal Cycler (Applied Biosystems). The amplification products were verified by agarose gel electrophoresis.
DNA sequencing
PCR products were sequenced in at least two independent amplification reactions using the reverse primer (5'-CCAATCAAACCCACAGACTTAC-3'). PCR amplicons were purified using isopropanol precipitation. Purified DNA was diluted and cycle sequenced using the ABI BigDye Terminator Kit v3.1 (ABI, Foster City, CA, USA) according to the manufacturer's instructions. Sequencing reactions were electrophoresed in an ABI3100 genetic analyzer. Electropherograms were analyzed by eye, and the sequences obtained were compared with the EZH2 reference sequence (GenBank NG_032043.1).
Statistical analysis
A comparison between cohorts was made with the Chi-squared test and Student's t-test, as required. RR was calculated along with their 95% CI, as a measure of risk to present CR or relapse. The logistic regression model was used to control confounders of the binary outcomes (CR or relapse), and odds ratio (OR) was calculated as a measure of association along with their 95% CI. The Kaplan–Meier method was used to construct survival curves, and the log-rank test was used for comparisons. The Cox's proportional hazards model was employed to define variables associated with PFS or OS and to control for confounders. Hazard ratios (HRs) were obtained as a measure of association along with their 95% CI.
A sample size of 188 patients was calculated assuming an expected RR of 3 for relapse, with 80% power and two-tailed probability value of 0.05. About 95% CI values and probability values of 0.05 or less were considered as significant, and two-tailed statistics were considered in all cases. SPSS version 25 software (IBM, Corp., Armonk, NY) was used for computations.
RESULTS
Patients
From January 2015 to December 2017, 218 patients with DLBCL attended the lymphoma clinic at this institution; of them, 20 patients were not included: 10 were treated with other chemotherapy regimens, 6 had HIV infection, and 4 did not accept to participate in the trial. Among these 198 patients, 93 were female (47%), and 105 were male (53%). The mean age was 58.4 years (standard deviation [SD] 14.1; range: 21-91 years).
Fifty patients had ≥ 2 ECOG performance status (25.3%); non-B symptoms were documented in 134 (67.7%); 79 had bulky disease (39.9%); 121 had extranodal disease (61.1%); 147 (74.2%) had Stages III and IV (Lugano classification); and 109 had intermediate-high or high IPI scores (55.1%). The germinal center (GCB) was the most frequent type (n = 137, 69.1%), 52 (26.3%) expressed MYC, and 45 had a double-hit lymphoma (22.7%). All patients were treated with 6 cycles of R-CHOP.
Mutations
All cases were analyzed for mutations at codon 641 in exon 16 of EZH2; 30 patients (15.1 %) had mutations, as follows: Y641N (n = 11, 5.5%), Y641F (n = 12, 6.1%), Y641H (n = 5, 2.5%), and Y641S (n = 2, 1%). There was one synonymous polymorphism in two patients (n = 2, 1%) and these two cases were included in the cohort of patients with wt EZH2. Figure 1 shows the electropherograms demonstrating these mutations. Table 1 compares the clinical and histopathological characteristics of the cohorts defined by wt EZH2 or EZH2 mutated. Main basal differences between cohorts were predominance of women, worse performance status by the ECOG scale, a higher proportion of patients within the high-risk category according to the IPI score in the cohort of patients with exon 16 EZH2 mutations. Except one case, all of those with mutated EZH2 were GCB by Hans algorithm, as expected (Table 1).
Table 1 Comparison of the clinical and pathological characteristics of patients with wt EZH2 and patients with EZH2 mutations
| EZH2 exon 16 wt and synonymous polymorphism (n = 168) | EZH2 exon 16 mutated (n = 30) | p | |
|---|---|---|---|
| Gender (female:male) | 74:94 | 19:11 | 0.051 |
| Age (years, mean, SD) | 58.5 (14.2) | 57.4 (14.1) | 0.676 |
| Hemoglobin (g/dL, mean, SD) | 14.3 (10.4) | 12.8 (2.1) | 0.432 |
| Serum albumin (g/dL, mean, SD) | 4.54 (7.4) | 3.77 (0.47) | 0.577 |
| Lymphocyte count (mean, SD) | 1,649 (1,166) | 1,297 (952) | 0.119 |
| Body mass index (mean, SD) | 26.6 (4.2) | 25.2 (3.3) | 0.093 |
| Prognostic nutritional index (mean, SD) | 45.4 (5.4) | 37.7 (4.7) | 0.577 |
| ECOG: n (%) | 131 (78) | 17 (56.7) | 0.005 |
| 0-1 | 35 (20.8) | 10 (33.3) | |
| 2-3 | 2 (1.2) | 3 (10) | |
| Presence of B symptoms | 51 (30.4) | 13 (43.3) | 0.162 |
| Bulky disease | 64 (38.3) | 15 (50) | 0.456 |
| Extranodal sites | |||
| None | 68 (40.4) | 9 (30) | 0.65 |
| 1 | 49 (29.1) | 8 (26.7) | |
| > 2 | 51 (30.5) | 13 (43.3) | |
| Lugano clinical stages | |||
| I-II | 46 (27.4) | 5 (16.6) | 0.501 |
| III-IV | 122 (72.6) | 25 (83.3) | |
| IPI | |||
| Low | 37 (22.0) | 4 (13.3) | 0.041 |
| Intermediate-low | 44 (26.0) | 4 (13.3) | |
| Intermediate-high | 35(21.0) | 4 (13.3) | |
| High | 52 (31.0) | 18 (60) | |
| Bone marrow infiltration | 10 (6) | 4 (13.3) | 0.146 |
| By Hans algorithm | |||
| GC | 108 (61.9) | 29 (96.7) | 0.009 |
| Non-GC | 60 (35.71) | 1 (3.3) | |
| MYC expression | 44 (27.7) | 8 (26.6) | 0.956 |
| Double-hit DLBCL | 30 (15) | 5 (16.6) | 0.875 |
| Response | |||
| CR | 132(78.5) | 19 (63.3) | 0.091 |
| PR | 14 (8.3) | 7 (23.3) | |
| SD + PD | 22 (13.1) | 4 (13.3) | |
| Relapsed | 32 (19) | 11 (36.7) | 0.031 |
| PFS (at 60 months, %) | 72 | 52.1 | 0.056 |
| OS (at 60 months, %) | 74.1 | 79.9 | 0.398 |
ECOG: Eastern Cooperative Oncology Group performance status; IPI: International Prognostic Index; GC: germinal center; non-GC: non-germinal center; CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; PFS: progression-free survival; OS: overall survival; p: probability value.
Clinical response
CR was observed in 151 patients (76.3%), partial response (PR) in 21 (10.6%), and stable disease or progressive disease (Deauville 4-5) in 26 (13.1%). The presence of any EZH2 mutation was negatively associated with CR after chemotherapy (RR 0.806, 95% CI 0.607-1.07, p = 0.3). The RR for the specific mutations of EZH2 and the outcomes of CR or relapse are shown in Table 1 suppl.
By bivariate analysis, CR was associated with basal platelet count (p = 0.041), basal serum albumin (p = 0.02), bulky disease (p = 0.033), presence of extranodal disease (p = 0.051), Lugano clinical stages (p = 0.024), and IPI score (p = 0.025). Multivariate analysis of independent factors associated with CR demonstrated that only basal serum albumin was significant (OR 1.938; 95% CI 1.112-3.378; p = 0.02).
Relapses
Relapses after complete response were found in 43 patients (21.7%). The frequency of relapses in the wt EZH2 cohort was significantly lower than in the cohort of patients with EZH2 mutations (RR 0.479, 95% CI 0.247-0.928, p = 0.031) (Tables 1 and 1 suppl).
By bivariate analysis, relapses were associated with the absolute lymphocyte count (p = 0.001), basal serum albumin (p < 0.0001), presence of B-symptoms (p = 0.025), Lugano stages (p = 0.014), IPI scores (p = 0.002), and subtype by the Hans algorithm (p = 0.037). Multivariate analysis demonstrated that Tyr641His (Y641H) and Tyr641Ser (Y641S), and basal serum albumin were independent factors associated with relapse (Table 2 suppl).
Survival
The median follow-up of both cohorts was 35.4 months (minimum of 0.36 months and maximum of 63.1 months; interquartile range of 29.9). During the follow-up of this study, 45 (22.7%) patients died and 14 patients (7.1%) were lost. The median PFS or OS has not yet been reached; thus, survival data are immature.
The bivariate associations of the presence of EZH2 mutations and PFS or OS are shown in Table 3 suppl. Patients with wt EZH2 presented marginally better PFS than patients with EZH2 mutated (HR 0.514, 95% CI 0.257-1.028, p = 0.06), and this association corresponds mainly to poor survival in the subgroups with Tyr641His (Y641H) and Tyr641Ser (Y641S) mutations (HR 3.234, 95% CI 1.149 – 9.1, p = 0.026) (Table 3 suppl).
The Kaplan–Meier PFS curves of cohorts are displayed in Figure 2.
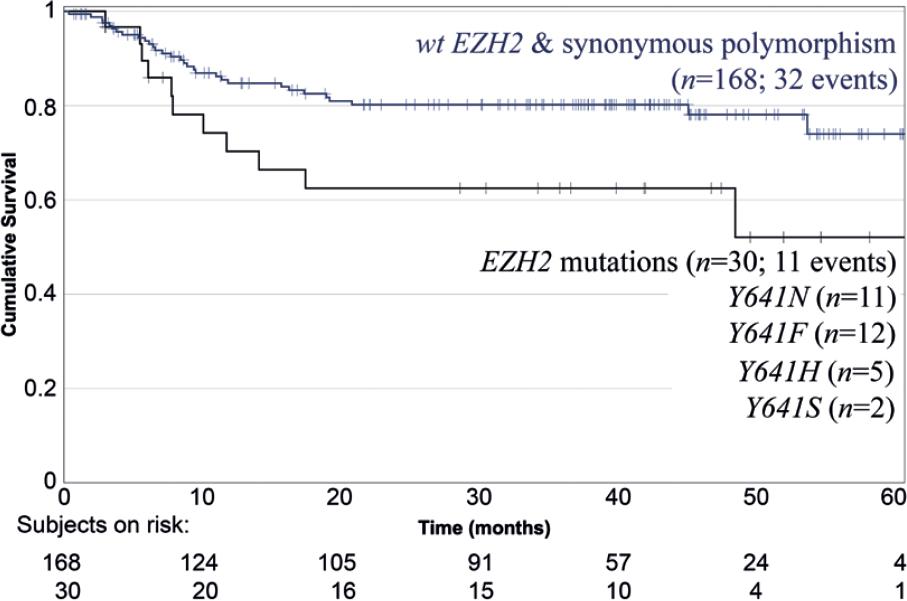
Figure 2 Kaplan–Meier progression-free survival in patients with wt EZH2 and synonymous polymorphism versus patients with EZH2 mutations (n = 198) (p = 0.031).
By multivariate analysis, only basal serum albumin was independently associated with PFS (HR 0.48; 95% CI 0.337-0.682; p < 0.0001). OS was not different in the cohorts with wt EZH2 or mutated EZH2, respectively (HR 1.495, 95% CI 0.584-3.824, p = 0.401).
DISCUSSION
The results of this study show that exon-16 EZH2 mutations were observed in 15% of cases in a Mexican-Mestizo population with DLBCL and that they were more frequent in the GC-B subtype. In addition, these mutations were more frequent in patients with poor ECOG and higher IPI scores but not with BCL2 or MYC expression. Interestingly, exon-16 mutations were associated with higher relapse/progression risk and showed a trend for not achieving complete response.
EZH2 is a 746 amino acid histone methyltransferase and is the catalytic subunit of PRC2, which catalyzes the trimethylation of lysine 27 on histone 3 (H3K27me3), a mark of transcriptional repression20. Different studies have evaluated the role of the EZH2 complex in both normal biology and tumorigenesis. B-cell differentiation has epigenetic control through EZH2 since pre-B cells require EZH2 to correctly undergo V(D)J recombination to produce functional immunoglobulins, and EZH2 expression is downregulated until B cells enter the germinal center reaction. This protein also influences on preserving a well-ordered GC-B development process21. In physiologic conditions, EZH2 silences antiproliferative genes, including CDK2 family cell cycle-related tumor suppressors (CDKN1A, CDKN1B, and CDKN2A)21.
The discovery of an activating somatic point mutation located at Y641 within the EZH2 catalytic SET domain, particularly a heterozygous missense somatic mutation replacing Y641 with asparagine, serine, histidine, phenylalanine, or cysteine in the SET domain, promotes the activation of EZH2, leading to increased levels of H3K27me322-25, suppressing expression of PRC2 target genes and a global redistribution of the tri-methylation mark26. Heterozygous mutation on tyrosine 641 is the most widely occurred EZH2 activating mutation in GCB-DLBCL and follicular lymphoma (FL).
The oncogenic effect of EZH2 gain-of-function mutations is to delay or block progression through the B-cell maturation step27. Gain-of-function mutations, as the described in this study, have been linked to the downregulation of tumor suppressor genes and differentiation, which allows for the emergence of additional genetic mutations and lymphomagenesis12,23,28. Furthermore, the Y641-mutated EZH2 is resistant to JAK2/BTRC-mediated degradation and is more stable than wt-EZH212. In addition, Velichutina et al. confirmed the oncogenic role of EZH228, demonstrating that downregulation of EZH2 mediated by siRNA in DLBCL cells resulted in cell cycle arrest at the G1/S transition and upregulation of tumor suppressor genes.
In populations from Canada, the United States, and France, approximately 20% of GCB DLBCL patients have activating EZH2 mutations13,14,24,29. These frequencies are higher than the reported in this study (15.2%). Our results are similar than those analyzed from the Phase III GOYA study30. In contrast, EZH2 mutations were detected only in 9% of patients included in the UK NCRI Molecular Profiling for Lymphoma (MaPLE) study31. These mutations have also been correlated with clinicopathological characteristics. Liu et al.13 investigated EZH2 expression and EZH2 Y641 mutations in 100 gastrointestinal DLBCL specimens by immunohistochemistry and sequencing. EZH2 was overexpressed in 50% of patients and was associated with advanced disease (0.014), reduced overall survival (p = 0.03), and higher International Prognostic Index score (p = 0.003). These authors also documented that EZH2 mutations had a significantly lower frequency (3.0%), than that in patients with DLBCL without gastrointestinal features. In our cohort study, we also had a higher proportion of patients with advanced disease (83.3%) within the EZH2 mutation group.
Other authors have reported that patients with primary central nervous system (CNS) DLBCL may also have a higher proportion, up to 30%, of EZH2 overexpression15. These results were not comparable with those from our cohort since we did not include patients with primary CNS DLBCL.
Our results agree with those reported by Oh et al.32 who suggested that patients with H3K27me (the product of EZH2) constitute another poor prognostic phenotype, that is, independent of MYC/BCL2 coexpression. In this study, we analyzed genetic variants in codon 641 at exon 16, and the number of patients with every mutation is low. However, after multivariate analysis, the presence of Tyr641His (Y641H) and Tyr641Ser (Y641S) mutations was an independent factor associated with relapse. The absence of other adverse histologically prognostic factors, such as double-hit lymphoma, MYC, or BCL2 expression in our mutant patients, is particularly interesting, since other oncogenic mechanisms or pathways require to be analyzed within the biology of DLBCL. In contrast with our results, in the MaPLe study, PFS was similar between mutated and unmutated patients31.
This finding is clinically relevant, as EZH2 mutations may constitute a target for therapy. In fact, tazemetostat, as a first-in-class selective inhibitor of EZH2, has been evaluated DLBCL type in cell cultures15. Recently, in a Phase I clinical trial, the use of tazemetostat produced responses in 8 of 21 patients with relapsed-refractory non-Hodgkin lymphoma33. A Phase Ib trial in first line, in previously untreated high-risk elderly patients with DLBCL, documented the feasibility of adding tazemetostat to R-CHOP, in terms of toxicity, where the most common adverse events were cytopenias and gastrointestinal toxicity34. A limitation of this study is the reduced number of patients. The real impact of EZH2 mutations on response rate, as it has been documented in other types of B-cell lymphomas35, requires validation in further studies.
This is the first study not only on DLBCL Mexican-Mestizo patients but also in Latin America that analyzes EZH2 mutations. Results of this study show that though exon-16 EZH2 gene mutations were associated with not achieving CR and presenting relapse/progression, longer follow-up and validation studies are required.











 nueva página del texto (beta)
nueva página del texto (beta)



