INTRODUCTION
Tuberculosis (TB) remains a significant global public health problem. In 2018, according to the World Health Organization (WHO), globally, an estimated 10.0 million (9.0-11.1 million) people became newly infected. The region of America informed around 3% of all reported cases1. In Colombia, TB represents an important public health problem; according to the Epidemiological Surveillance System of the National Institute of Health (INS), there were 14,338 cases notified in 2018, for an incidence of 26/100,0002. TB is localized in segments of the most vulnerable population, such as HIV patients (11 out of 100), indigenous people (5 out of 100), street dwellers (4 out of 100), and inmates (6 out of 100). Besides, the burden of the disease is mainly condensed in cities such as Medellín, Cali, Bogotá, Barranquilla, and Villavicencio3. Medellín is the second-largest city in the country, with a population of approximately 2.5 million inhabitants. Since 2007, this municipality has had higher incidences than the national average, around 60/100,0002,3.
Although the pulmonary form of TB is the most frequent, extrapulmonary TB (ETB) represented 15% of incident cases notified in 2018 worldwide1. In Colombia, the ETB rate was 16.7% in 2017, and its frequency of presentation is markedly increased in cases of immunodeficiency4. A definitive diagnosis of TB and ETB can only be made by culturing Mycobacterium tuberculosis organisms from a specimen obtained from the patient; however, extrapulmonary samples are a challenge due to their low bacillary burden and the variety of presentations5.
A comprehensive evaluation of a patient suspected of having contracted TB includes clinical history, physical examination, chest X-rays, and microbiological tests. Conventional smear microscopy has a low sensitivity (0-40%). Mycobacterial culture sensitivity differed in the previous studies by site of ETB: 28-50% for abdominal TB, 10-11% for TB pericarditis, 24-29% for TB meningitis, and 5-14% for TB lymphadenitis5. Furthermore, it usually takes 2-8 weeks to obtain culture results. Genotypic tests by polymerase chain reaction (PCR) have been commercially developed based on M. tuberculosis identification and detection of mutations associated with rifampin (RIF) resistance. One of these tests is the Xpert MTB/RIF (Cepheid)® assay, the first point-of-care assay for TB, which was endorsed by the WHO in 20106; since then, it has become a breakthrough in TB diagnosis.
In recent years, an upgraded version of the assay has been developed, called Xpert® MTB/RIF Ultra (Xpert/Ultra), with improved sensitivity for the detection of M. tuberculosis and RIF resistance-determining region of the rpoB gene. It includes detection of the new semi-quantitative category called "trace," which enables the assay to detect from 16 CFU/mL, compared to 114 CFU/mL of the previous version. Furthermore, it incorporates two different multicopy amplification targets, IS6110 and IS10817. Xpert/Ultra represents a sensitive, rapid, and reasonable technique that does not require complex infrastructure, ideally for a limited economic resources setting. To date, unlike the other molecular assays, Xpert/Ultra has been evaluated on extrapulmonary samples in a restricted number of studies, particularly in Colombia. Thus, the objective of this study was to assess the usefulness of Xpert/Ultra to detect extrapulmonary infection by M. tuberculosis under routine diagnostic conditions in patients with suspected ETB from a tertiary referral center.
METHODS
Study setting and population
A descriptive retrospective study was performed at the Hospital Universitario San Vicente Fundación (HUSVF), between July 2019 and July 2020. The HUSVF is a University Teaching Hospital located in Medellín, Colombia, and is a reference center for infectious diseases, with 819 beds of total inpatient capacity. This study is based on a convenience sample. The inclusion criteria were all clinical extrapulmonary specimens that underwent microbiological examination, which included Ziehl–Neelsen (ZN) technique, culture, and molecular test. There was no age restriction. Exclusion criteria included incomplete medical records, technical issues, and culture contamination.
Clinical, microbiological, and sociodemographic information
The medical history of each patient was obtained from clinical charts. The following variables were analyzed: sex, age, HIV status, patient comorbidity, and microbiology laboratory results.
Microbiological methods
Extrapulmonary samples were prepared for microscopic examination. The specimen was cultivated on both, solid Löwenstein–Jensen medium (LJ) and liquid medium using the MGIT 960 system (Becton Dickinson Diagnostic Systems®), according to the manufacturer's instructions. Two methods were used to increase sensitivity. Samples were incubated until they became positive or for a maximum of 40 days to deliver a negative result. In positive cultures, M. tuberculosis was identified in grown colonies using SD Bioline TB Ag MPT64 assay (Standard Diagnostics, Yongin-si, Gyeonggi-do, Republic of Korea; hereafter, SD MPT64 [assay]), according to the manufacturer's guidelines; then, cultures were tested for identification and susceptibility to first-line anti-TB drugs, isoniazid and RIF, at the regional reference mycobacteria laboratory by GenoType MTBDRplus line probe assays (Hain Lifescience, Nehren, Germany).
Preparation of clinical samples for molecular assay
Biopsy specimens were divided into small fragments and resuspended in 3 mL of sample reagent (SR), provided by the diagnostic kit to inactivate and liquefy the specimen. Resuspended specimens were then transferred to a Falcon tube, and the mixture was vigorously shaken for at least 10 s. Fluids (pleural, ascitic, and urine) with a volume of 2 mL or more were concentrated by centrifugation at 3000 g for 15 min, and the supernatant fluid was discarded; sediment was suspended into 2.5 mL of SR. Samples with a volume of <2 mL were gauged to a minimum of 2.5 mL with SR. The tube containing the sample was vortexed for at least 10 s and incubated at room temperature for 10 min; the mixture was vortexed for another 10 s and incubated at room temperature for 10 min. Finally, in all samples, 2 mL were pipetted into the cartridge and inserted into the GeneXpert Dx instrument for PCR testing. The measurement fluorescent signal and embed calculation algorithms were conducted automatically by the GeneXpert Dx System software. It is important to clarify that the same sample was used to perform the conventional tests and Xpert/Ultra.
Statistical analysis
For the descriptive analysis, qualitative approaches were presented as percentages and absolute frequencies, while quantitative variables were described using central tendency measures. Kappa test, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and positive and negative likelihood ratios (LRs) with 95% confidence intervals (CI) were calculated. For the study purposes, both BACTEC MGIT 960 and LJ cultures were used as the reference methods. All data were analyzed using EPIDAT 3.1 (Consellería de Sanidade, Xunta de Galicia, España) and R (version 3.6.3, The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
A total of 540 consecutive samples from patients with suspected ETB were included, from which 18 were excluded due to contamination. In addition, during the study period, three samples were unsuccessful due to flaws in the internal control (invalid result). Thus, 519 specimens from 432 patients met the inclusion criteria for analysis. In general, there was a predominance of male patients, with 58.1% (251/432). The median age was 44 years (interquartile range: 27-63), ranging from 1 month to 92 years of age.
M. tuberculosis was detected in 10.2% (53/519) of specimens by Xpert/Ultra, whereas culture was positive in 7.1% (37/519) (Fig. 1). In contrast, five samples presented discordant results between culture and molecular method. On the other hand, only five samples were positive on ZN smear: gastric aspirate (three cases), lymph node (one case), and lung biopsy (one case); all of them were detected by Xpert/Ultra.
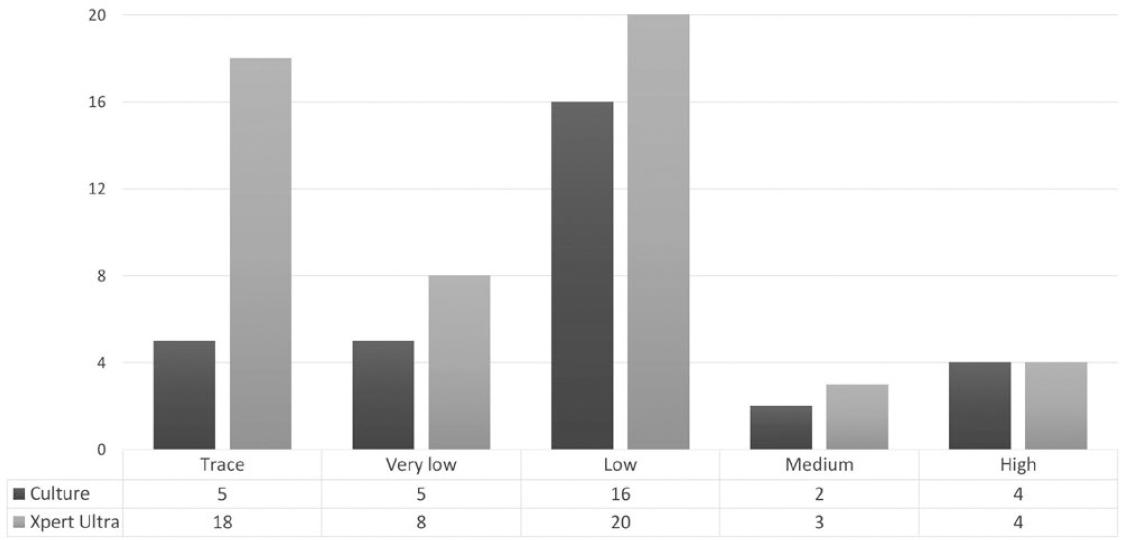
Figure 1 Distribution of positivity in culture versus Xpert® MTB/RIF Ultra according to semi-quantitative categories of extrapulmonary samples.
Among positive patients (46/432), six were positive in two (five cases) and three (one case) different samples. The diagnosis of HIV was present in 26% (12/46), and 36.9% (17/46) had a history of pulmonary TB. Other general descriptive data of the patients are shown in Table 1.
Table 1 Baseline sociodemographic and clinical characteristics of patients with positive test by Xpert® MTB/RIF Ultra according to culture result
| Variables | Total | Xpert/Ultra positive | |
|---|---|---|---|
| Culture+ | Culture− | ||
| 46 | 26 | 20 | |
| Demographic data | |||
| Male | 29 | 18 | 11 |
| Female | 17 | 8 | 9 |
| Age group (years) | |||
| 0–15 | 9 | 7 | 2 |
| 15–30 | 12 | 5 | 7 |
| 31–45 | 16 | 12 | 4 |
| 46–60 | 3 | 0 | 3 |
| 61–75 | 4 | 1 | 3 |
| 76–92 | 2 | 1 | 1 |
| History of pulmonary TB | |||
| No | 29 | 18 | 11 |
| Yes | 17 | 10 | 7 |
| HIV infection | |||
| No | 34 | 19 | 15 |
| Yes | 12 | 9 | 3 |
| Comorbidities | |||
| Lymphoma | 5 | 3 | 2 |
| CRD | 3 | 0 | 3 |
| Diabetes | 2 | 0 | 2 |
TB: tuberculosis; HIV: human immunodeficiency virus; CKD: chronic kidney disease.
Overall, estimated sensitivity of Xpert/Ultra was 86.49 (95% CI, 74.12-98.85), specificity was 90.6% (95% CI, 93.72-97.57), PPV 60.3% (95% CI, 46.2-74.4), NPV 98.9 (97.8-99.9), +LR 19.8 (95% CI, 12.8-30.7), and -LR 0.14 (95% CI, 0.06-0.32). The sensitivities among different sample groups are shown in Table 2. The agreement value of Kappa index was 0.6846 (95% CI: 0.57-0.79).
Table 2 Operative characteristics of Xpert® MTB/RIF Ultra using mycobacteria growth indicator tube (MGIT)/Löwenstein – Jensen culture as standard reference methods according to the type of specimen
| Specimen | Total | Culture positive | Culture negative | Sensitivity% (95% CI) | Specificity% (95% CI) | PPV% (95% CI) | NPV % (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|
| Xpert + | Xpert – | Xpert + | Xpert – | ||||||
| CSF | 204 | 12 | 3 | 8 | 181 | 80.57 (56.42-100) | 95.77 (92.63-98.90) | 60 (36.03-83.97) | 98.37 (96.27-100) |
| Urine | 103 | 1 | 0 | 5 | 97 | 100 (50-100) | 95.10 (90.42-99.78) | 16.67 (0-54.82) | 100 (99.48-100) |
| Pleural fluid | 93 | 3 | 0 | 6 | 84 | 100 (83.33-100) | 93.33 (87.62-99.04) | 33.33 (0-69.69) | 100 (99.40-100) |
| Ascitic fluid | 46 | 2 | 1 | 0 | 43 | 66.67 (0-100) | 100 (98.84-100) | 100 (75.00-100) | 97.73 (92.19-100) |
| Gastric aspirate | 19 | 5 | 0 | 0 | 14 | 100 (90.00-100) | 100 (96.43-100) | 100 (90.00-100) | 100 (96.43-100) |
| Pericardial fluid | 15 | 0 | 0 | 0 | 15 | NA | NA | NA | NA |
| Lymph node | 11 | 5 | 0 | 1 | 5 | 100 (90-100) | 83.33 (45.18-100) | 83.33 (45.18-100) | 100 (90-100) |
| Synovial fluid | 6 | 0 | 0 | 0 | 6 | NA | NA | NA | NA |
| Lung biopsy | 6 | 2 | 1 | 1 | 2 | 66.67 (0-100) | 66.67 (0-100) | 66.67 (0-100) | 66.67 (0-100) |
| Vertebral biopsy | 3 | 1 | 0 | 0 | 2 | 100 (50-100) | 100 (75-100) | 100 (50-100) | 100 (75-100) |
| Pleural biopsy | 3 | 0 | 0 | 0 | 3 | NA | NA | NA | NA |
| Skin biopsy | 3 | 0 | 0 | 0 | 3 | NA | NA | NA | NA |
| Brain biopsy | 2 | 0 | 0 | 0 | 2 | NA | NA | NA | NA |
| Joint biopsy | 1 | 0 | 0 | 0 | 1 | NA | NA | NA | NA |
| Colon Tissue | 1 | 0 | 0 | 0 | 1 | NA | NA | NA | NA |
| Bone marrow | 1 | 0 | 0 | 0 | 1 | NA | NA | NA | NA |
| Lung aspirate | 1 | 1 | 0 | 0 | 0 | NA | NA | NA | NA |
| Liver biopsy | 1 | 0 | 0 | 0 | 1 | NA | NA | NA | NA |
| Total | 519 | 32 | 5 | 21 | 461 | 86.49 (74.12-98.85) | 95.64 (93.72-97.57) | 60.38 (46.27-74.49) | 98.93 (97.88-99.97) |
PPV: positive predictive value; PNV: predictive negative value; CSF: cerebrospinal fluid; NA: not applicable. NPV: negative predictive value; CI: confidence intervals.
Among the 53 samples positive by Xpert/Ultra, 31 were susceptible to RIF; 17 were indeterminate; and one was resistant. Of them, 32 samples had a positive culture and were analyzed to RIF-IHN susceptibility by the phenotypic method. There was an agreement of 100% for 26 isolates sensitive to RIF, while six remaining indeterminate were found to be susceptible to RIF.
Regarding the semi-quantitative categories by Xpert/Ultra, the most frequent load found was "low" in 20 cases followed by "trace" in 18 cases. On overage, the general turnaround time required for a positive culture was 25.8 ± 8.94 days (Table 3).
Table 3 Characterization of positive detections in Xpert® MTB/RIF Ultra by semi-quantitative values with other relevant parameters
| Total | Trace | Very low | Very low | Low | Low | Medium | High | ||
|---|---|---|---|---|---|---|---|---|---|
| RIF indeterminate | RIF indeterminate | RIF S | RIF S | RIF R | RIF S | RIF S | |||
| (n = 18) | (n = 1) | (n = 7) | (n = 19) | (n = 1) | (n = 3) | (n = 4) | |||
| Positivity culture | n | 32 | 5 | 1 | 4 | 15 | 1 | 2 | 4 |
| Ct | Time days (SD) | 25.8 (8.9) | 27.2 (9.5) | 29 | 28.5 (5.19) | 27.1 (10.86) | 26 | 19.5 | 19 (3.16) |
| SPC (DE) | 26.3 (2.8) | 26.16 (2.8) | 24.4 | 25.5 (0.8) | 25.8 (3.0) | 25.9 | 25.9 (1.4) | 30.8 (1.1) | |
| IS6110-IS1081 (DE) | 22.1 (4.1) | 26.4 (2.6) | 22.2 | 22.9 (1.5) | 19.9 (1.8) | 19.9 | 16.4 (0.1) | 16.1 (0.1) | |
| Type of sample (53 cases) | CSF | 20 | 8 | 1 | 3 | 8 | 0 | 0 | 0 |
| Pleural fluid | 9 | 7 | 0 | 1 | 1 | 0 | 0 | 0 | |
| Lymph node | 6 | 1 | 0 | 2 | 2 | 0 | 0 | 1 | |
| Gastric aspirate | 5 | 0 | 0 | 0 | 2 | 0 | 1 | 2 | |
| Urine | 6 | 2 | 0 | 1 | 2 | 0 | 1 | 0 | |
| Ascitic fluid | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | |
| Pus | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Vertebral biopsy | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| Lung biopsy | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | |
Rif: rifampin; S: sensitive; R: resistant; SPC: control; SD: standard deviation; Ct: cycle threshold; CSF: cerebrospinal fluid.
DISCUSSION
An early diagnosis is the first step toward a better management of TB. The present study attends to an urgent need for evidence on a user-friendly and reliable molecular diagnostic test for TB in extrapulmonary specimens, in the context of a developing country. To the best of our knowledge, this is the first report on the use of Xpert/Ultra with consecutive inclusion of samples in a clinical routine setting in Colombia.
Most of the positive cases for ETB were men (63%), ranging from 1 month to 94 years of age; these findings are in line with data from the WHO since people in all age groups are affected by TB, although the highest burden is among adult men1. In relation to comorbidities, one-quarter of positive cases had concomitant HIV infection. This is also consistent with the fact that ETB has reemerged in the past years, with a majority of cases in regions with high HIV prevalence. According to the WHO, the use of Xpert/Ultra is endorsed in people with suspected ETB and those living with HIV because both diagnoses are extremely challenging. On the other hand, five cases presented with both ETB and lymphoma, which could be quite similar in clinical presentation and radiological findings, a fact that has been reported to hinder the final diagnosis8. Other prevalent factors were chronic renal disease in three patients and diabetes in two patients, both of which are associated with ETB9. Xpert/Ultra was useful as a diagnostic tool in those cases because cultures were negative.
In the present study, Xpert/Ultra had an overall sensitivity of 86.4%, and this value varied according to the sampling site. Wu et al. evaluated the diagnostic performance of Xpert/Ultra on various types of samples (cerebrospinal fluid [CSF], lymph nodes, bone, and urine); the overall sensitivity in cases with suspected ETB was 83.7% and specificity was 92.0%10. A recent systematic review reported 85.1% (95% CI: 76.7-90.8%) sensitivity and 95.7% (95% CI: 87.9-98.6%) specificity in seven studies11. Among the reports comparing with a previous version of the molecular test, Wang et al. demonstrated that Xpert/Ultra had a higher diagnostic sensitivity than Xpert MTB/RIF (44.19% vs. 18.60%, p = 0.011)12. The increase in sensitivity is due mainly to the incorporation of two new targets (IS1081 and IS6110). However, in several studies, the specificity of Xpert/Ultra was lower than Xpert MTB/RIF in extrapulmonary specimens10,12,13.
Regarding the type of sample, CFS was more predominant during the study period; it had a sensitivity of 80.5% and a specificity of 95.7%. The previous studies have reported sensitivities ranging from 59.5% to 72.05%, and specificities around 100%14-17. Sensitivity can be affected by the volume of CSF tested and by whether CSF was centrifuged before testing18. According to the previous findings, the use of fresh or frozen CSF samples, with a proper cryopreservation, does not alter the yield of detection of M. tuberculosis17. Furthermore, it has been reported that Xpert/Ultra was not superior to the version of Xpert MTB/RIF in CSF of individuals with tuberculous meningitis, using either clinical or culture reference standards16. In this study, the positive results were determinant for clinical decisions (including the "trace" category); likewise, with a mortality approaching 100% in untreated tuberculous meningitis, it is less probable that the trace category could have been due to previous infection17. Therefore, these data highlight the importance of routine molecular testing of CSF; Xpert/Ultra is valuable when it is positive, and any load should be considered.
Regarding lymph nodes, only five samples were positive. Results of other studies have reported a high sensitivity in these specimens (90-94%)19,20; an explanation could be that tissues and abscesses have higher bacillary loads than other samples10. With respect to pleural TB, in a recent report that included a large cohort study, Xpert/Ultra had a positivity of 44.23%, demonstrating a higher sensitivity than both culture (26.44%) and a previous version of the Xpert assay (19.23%)21. In the present work, M. tuberculosis was detected in six additional cases in contrast with culture.
An important aspect of Xpert/Ultra is the semi-quantitative classification; in this study, we found that one-third (33.9%) of positive cases were classified in the "trace" category, the lowest bacillary load. Despite the software cannot provide information about RIF resistance, it is relevant in the clinical context of the patient. The Global Laboratories Initiative (guidelines), and the working group of Stop TB Partnership, states that "trace category should be considered as a true-positive result for use in clinical decisions and patient follow-up in case of extrapulmonary samples22." However, there is also uncertainty surrounding the interpretation of this category. It is important to consider that the mean copy number of the multicopy amplification target IS6110 varies between M. tuberculosis of different lineages, which might affect Xpert/Ultra detection in different countries. The Beijing family belongs to the East Asian lineage (Lineage 2). For example, the L2 lineage has the highest mean copy number of IS611023; this is the predominant lineage found in Vietnam24. The L4 lineage, predominant in Africa, shows large variation in IS6110 mean copy numbers25. In the America region, the prevalence of the Beijing lineage detected can vary widely: Perú (5.9%), Argentina (1.0%), Brazil (0.8%), and Paraguay (0.6%)26. The Beijing lineage in Colombia has been reported in small numbers27; therefore, the above suggests that this feature should be taken into account when determining the performance of the molecular test.
Regarding false positives, this scenario could be due to the paucibacillary nature of the disease. Although culture is considered the best reference standard for TB diagnosis, it may misclassify cases of ETB as negative. The molecular test with improved sensitivity, but with reduced specificity, potentially detects small numbers of non-viable bacilli, which can ultimately lead to "trace" readings or false-positive results. This could be a limitation to its use in some clinical cases, especially for the trace semi-quantitative category in patients previously treated for TB or with remaining DNA from old infections7,28,29. Another factor to be considered, despite the automatization of Xpert/Ultra, is that, as in any molecular assay, good laboratory practices must be followed for sample separation and processing. On the other hand, regarding false-negative results, in this study, there were five cases; it is necessary to consider the incorrect processing of the sample during homogenization and decontamination (smear, culture, and molecular test), because this can result in an insufficient number of detectable bacilli that can be suitable for culture, but that could have reduced the sensitivity of the Xpert/Ultra. In these cases, only one patient had HIV infection; one had systemic lupus erythematosus, and the remaining three patients had no comorbidities. Thus, in all suspected cases, culture should be performed at a minimum, and Xpert/Ultra should be used in addition to conventional tests and clinical information.
There are limitations to this study. First, there were a few numbers of specimens such as synovial fluid, bone marrow, and biopsies; larger studies with those types of samples are needed to assess performance. Second, the low number of RIF resistant strains detected in this study does not allow a proper assessment of susceptibility tests. Third, it was not possible to consider TB treatment, which may influence culture interpretation. However, this is a retrospective study with a large consecutive inclusion of samples in a clinical context from a setting with intermediate TB prevalence, which can provide useful information about assay performance on extrapulmonary samples for the Latin America region.
Finally, as the main contribution, the molecular assay had a significant proportion of cases detected (21 samples) that were not otherwise diagnosed by traditional tests (ZN and culture), representing a remarkable improvement. In summary, the implementation of Xpert/Ultra is extremely simple, making this molecular assay suited for any laboratory performing detection in extrapulmonary samples. These findings suggest that Xpert/Ultra is a useful complementary test that can contribute to a rapid diagnosis of ETB in our setting and can be generalized to other populations. Xpert/Ultra has potential advantages for its application in decentralized or smaller laboratories, and it is an evident molecular tool for the diagnosis of ETB.











 nueva página del texto (beta)
nueva página del texto (beta)


