Several metabolic disorders are associated with changes in dietary patterns, leading to obesity and a number of comorbidities, including insulin resistance, hyperlipidemias, hypertension, and fatty liver, among others1. Therefore, the type of nutrients consumed in the diet can promote or attenuate these abnormalities. To maintain adequate nutritional status, several countries, based on the clinical and metabolic consequences of nutritional deficiencies, established 80 years ago nutritional recommendations for different nutrients to prevent or avoid diseases related with a nutritional deficiency2. However, with the emergence of the obesity epidemic, it has been considered that all subjects from different populations have a similar metabolic response to nutrients, establishing that nutritional recommendations are essential to prevent obesity and its comorbidities. Even so, despite these efforts, the problems associated with obesity are still present in our society.
Interestingly, in the last decade, it has been demonstrated that there is a great variability in the population in the effects associated with nutrient intake, particularly the main macronutrients carbohydrates, fats, and proteins3. This variability modifies the response to several factors such as energy expenditure, postprandial glycemia, circulating levels of lipids, particularly fatty acids, triglycerides, and cholesterol, among others. These results suggest that individual variation in the response to nutrients has not been considered, indicating that the nutritional care for each subject must be individualized, leading in recent years to a new concept in nutrition, called personalized nutrition or precision nutrition (Fig. 1).
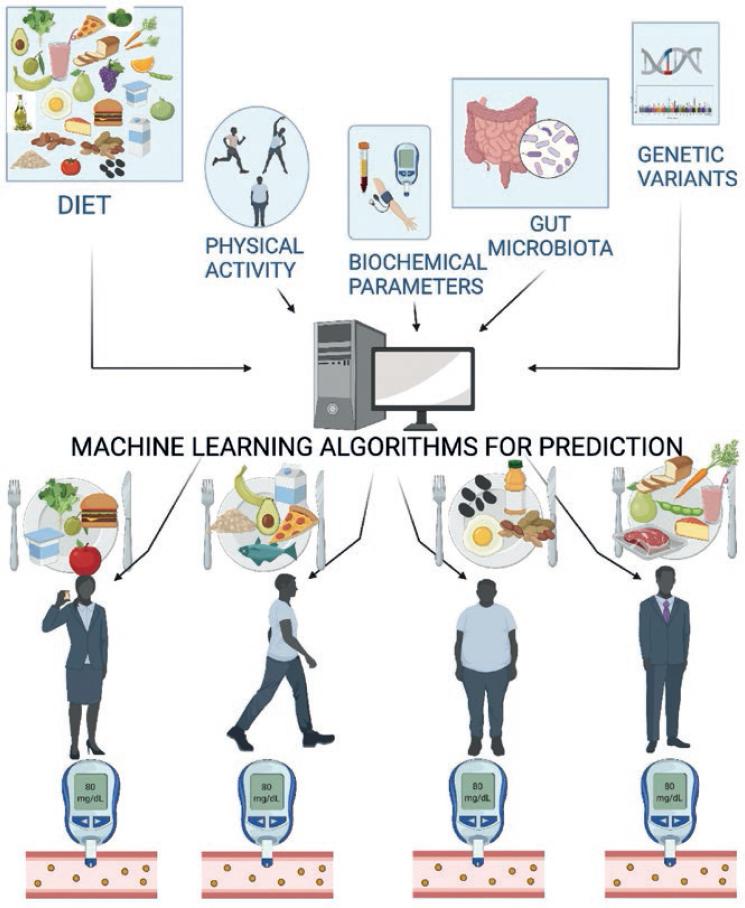
Figure 1 Personalized nutrition or precision nutrition. Information obtained from diet, physical activity, blood biochemical parameters, genetic variability, and gut microbiota are integrated into the algorithm that allows prediction of an individual’s metabolic response, for example, to postprandial glucose peaks based on foods that are selected on a personalized basis to keep the variability of the response within normal levels.
However, efforts have been focused on determining which factors should be considered to establish the individuality of the response to a nutrient, a food, or a diet. In the early 2000s, with molecular biology technologies, a new area of nutrition was developed, called nutritional genomics, to understand how nutrients regulate the metabolic response. Nutritional genomics has two aspects that have been studied in great detail in the past three decades, nutrigenomics and nutrigenetics4. Nutrigenomics studies the mechanism of action of nutrients at the molecular level and how they regulate gene expression, including responses at the transcriptional, translational and post-translational levels. It is now understood how many nutrients can selectively regulate gene expression for cellular utilization of these dietary components5. On the other hand, the aim of nutrigenetics is to determine how the organism responds to different nutrients based on genetic variants in the genome called polymorphisms4. This has explained, in part, why there are individuals who are hyper-responders, normo-responders, or hypo-responders to the metabolic effects generated by the consumption of specific nutrients or foods6. Based on the concepts of nutritional genomics, nutritional strategies have been designed to address metabolic disorders associated with obesity.
Although it was thought that genetic variability could demonstrate how it might influence an individual’s metabolic response to a nutrient or food relative to other people, twin population studies showed that genetics was not the only factor affecting the response to a specific diet. It was observed that in several cases, particularly in twins, despite their genetic similarity, there was an additional factor that generated changes in their metabolic responses, because one twin could benefit from one type of diet, and the other could not7. By 2015, a new factor, the gut microbiota, was incorporated to explain the changes in metabolic response observed between different individuals8.
The microbiota is defined as the bacterial community that inhabits a specific environment. Therefore, there are several microbiota in the body located in the skin, mouth, nose, vagina, and intestine, among others9. With the advent of next-generation DNA sequencing technologies, it has been possible to study in greater detail the taxonomy of the microbiota in different environments. In particular, in the gut there are between 1300 and 1400 species, which are classified into specific phyla, including mainly the Bacteroidetes, Firmicutes, Proteobacteria, Verrucomicrobia, and Actinobacteria, although there are some other phyla. Bacteroidetes and Firmicutes account for over 80% of the bacteria in the gut microbiota. Studies from 2004 to date have shown that the gut microbiota has a major influence on the development of obesity, but it depends on the type of bacteria colonizing the gut10. The type of bacteria present in the gut microbiota depends on multiple factors, including the type of birth, whether it was vaginal delivery or by cesarean section11, consumption of antibiotics12, and treatment with some drugs13, among others. However, the main factor that modifies the gut microbiota is the diet, and it has been shown that the intake of diets high in simple carbohydrates and high in fats generates an imbalance in the gut microbiota called dysbiosis14. Dysbiosis of the gut microbiota has been shown to generate low-grade inflammation due to metabolic endotoxemia, leading to abnormalities in carbohydrate, and lipid metabolism15.
In recent years, the influence of various factors on postprandial blood glucose has been studied. An excessive frequency of elevated postprandial blood glucose peaks has been shown to be the main determinant for the development of type 2 diabetes and cardiovascular disease16. Analyses of the studies conducted so far indicate that the factors affecting postprandial blood glucose peaks are the composition of the food consumed, especially if it is abundant in carbohydrates; to a lesser degree, the person’s genetics; although, very importantly by the person’s gut microbiota17. At present, postprandial glucose peaks have been considered to depend almost exclusively on the carbohydrate load of the foods consumed or their glycemic index; however, the influence of these factors is not absolute, as these studies suggest that the type of gut microbiota is a very important factor determining the variance of the postprandial peak glucose response. In fact, based on the information on the diet consumed by individuals, their blood metabolic variable profiles, anthropometric variables, physical activity and gut microbiota, by machine-learning algorithms, are allowing to predict which type of diet an individual should consume to decrease significantly the postprandial peaks of glucose8. This is the beginning of the real conceptualization of what will be personalized or precision nutrition, since by integrating all the variables that contribute to a beneficial metabolic response, it will be possible to provide people with an individualized recommendation of the type of food to be consumed depending on all the variables analyzed and integrated.
It is important to consider that people’s diets can vary substantially from one country to another, or even within a country from one region to another, which can significantly modify the gut microbiota18,19. Consequently, personalized nutrition, as its name suggests, cannot be generalized due to the variability of the gut microbiota. However, one of the most promising lines of research is to establish within the gut microbiota which specific bacteria influence a metabolic response. Efforts have been initiated to discern which bacteria generate a particular effect on the organism, and of these, bacteria are being studied to determine the metabolic pathways that are most active, and which metabolites are produced, that may influence the host response. This new area of research looking for novel metabolites produced by bacteria of the gut microbiota, known as metabolomics, will shed light on why certain bacteria exert beneficial health effects20.
On the other hand, with the emerging knowledge about the gut microbiota and which types of bacteria generate beneficial effects, another very important aspect to reach a personalized nutrition is to modulate the gut microbiota through the diet using foods that have properties which allow modifying the taxonomy of the gut microbiota to produce metabolic responses that selectively improve the patient’s metabolism21. It is important to mention that nutrigenomics and nutrigenetics have already established how different nutrients modulate gene expression in a selective manner, allowing for food combinations that contain nutrients which synergistically stimulate one or more gene expression pathways leading to a desired metabolic effect. These food combinations, known as dietary portfolios or dietary patterns, have shown benefits in carbohydrate or lipid metabolism in patients with obesity or metabolic syndrome22,23.
The next step is to establish not only how nutrients or foods influence aspects of nutrigenomics and nutrigenetics but also to integrate into this knowledge how these nutrients or foods can modify the gut microbiota. This type of research should be emphasized by studying regional foods that are frequently consumed by the local population, since some of these foods are only consumed in one country or region. At present, the effect of different types of sweeteners and polysaccharides in the diet, as well as different types of dietary fats or oils and dietary proteins are being studied. Furthermore, there has been a growing interest in the effects of dietary bioactive compounds, since it has been shown that these molecules present in numerous foods have an important impact on the gut microbiota and the host metabolism.
An interesting aspect to be included in personalized nutrition is the thermogenesis of individuals, particularly associated with adaptive thermogenesis, which allows to increase energy expenditure. This increase in energy expenditure is an aspect of great relevance at present to prevent the positive energy balance observed during obesity. Adaptive thermogenesis is associated with an increase in brown adipose tissue activity, as well as an increase in the differentiation of white adipose tissue into beige adipose tissue. Current studies demonstrate that there are dietary bioactive compounds that stimulate thermogenesis through the activation of brown and beige adipose tissue. Recently, it has been shown in experimental animals that the gut microbiota can stimulate thermogenesis through the activation of brown adipose tissue and the conversion of white adipose tissue into beige adipose tissue, a process known as browning, thus nutrients or foods that selectively modify the gut microbiota could stimulate thermogenesis and in consequence, decrease body weight.
One of the aspects that are contributing to an improved approach to personalized nutrition is the use of continuous monitors to determine over several days the fluctuation of biochemical variables such as glucose concentrations throughout the day and night. The development of new monitors to assess variations in lipids, such as circulating free fatty acid levels, may in the future establish a better relationship between these and the diet, gut microbiota, and other metabolic parameters.
CONCLUSIONS
The basis of personalized nutrition or precision nutrition is breaking several paradigms of traditional nutrition, with the objective of providing patients with improved dietary strategies to prevent metabolic deterioration of the individuals. Much more research is still needed to establish predictive algorithms to achieve these goals. However, a very attractive future is envisioned for the field of nutrition to reduce the burden on the health sector of the consequences of obesity and its comorbidities.











 nueva página del texto (beta)
nueva página del texto (beta)


