INTRODUCTION
Healthcare-associated infections (HAIs) are the most frequent adverse events related to patient care1, but they can be avoided when adhering to prevention practices2,3. Use of prevention bundles4 in our hospital led to a gradual improvement in the prevention of ventilator-associated pneumonias (VAPs)5 and bloodstream infections (BSIs) (not published), achieving nearly zero rates in months previous to the severe acute respiratory syndrome coronavirus 2 pandemic. Full COVID-19 hospital surge capacity response (HSCR) started on March 16, 2020, and with it came the following changes in clinical workflow: (1) the nurse-to-patient ratio in the intensive care unit (ICU) declined from 1:1 to 1:2 during HSCR due to bed expansion; (2) <10% of staff was specialized in critical patient care; (3) 4 h shifts were introduced to avoid fatigue related to the use of personal protective equipment; and (4) prone positioning and infusion of muscle relaxants were fully implemented during HSCR, and daily awakening protocols were reduced as a consequence. In addition, epidemiological surveillance rounds were temporarily restricted by heads of different hospital areas in an attempt to lower overall risk of contagion among staff.
It was unknown if these changes had an impact on HAI rates. Therefore, the objective of this research was to calculate HAI rates before and after HSCR, as well as to study the most likely causes of any differences.
METHODS
A before-after observational study was conducted. Active search and report of HAIs as well as patient predisposing factors are routinely done in a prospective, standardized manner and did not undergo any changes during the study period. Mainly due to this reason, the institutional review board granted a waiver for informed consent; furthermore, the study was deemed to pose no risk to patients due to the use of deidentified data in the database.
Our institutional Infection Prevention and Control (IPC) program relies on the following: (1) daily prospective surveillance, (2) HAI prevention bundles, (3) antibiotic stewardship, (4) continuing education, and (5) performance feedback on a monthly basis. Objective evidence on the conduct of these activities is routinely recorded on standardized sheets and reported monthly, as per hospital policies.
Starting in May 2020, additional IPC measures instituted were (1) retraining in prevention bundles, (2) retraining in safe central venous catheter insertion practices, (3) halting of double gloving, (4) emphasis on hand hygiene, and (5) weekly meetings between hospital authorities and heads of clinical areas.
HAIs were identified in all hospitalization areas (emergency department, ICU, accessory critical area, and hospital wards) and defined according to the NHSN standardized criteria current in 2019 and 20206. HAI events and the corresponding denominator data were recorded daily. Monthly HAI rates were calculated dividing the number of events by the number of patient-days and then multiplying by 1000; patient-days was used to simplify comparisons between different types of HAIs (rates adjusted to device-days and patient-days gave similar trends).
The following variables were compared among patients affected by HAIs: sex, age, length of stay before diagnosis of HAI, hospital area where HAI was identified, rate of in-hospital death, risk factors for hospital-acquired pneumonias and VAPs (endotracheal intubation, tracheostomy, use of humidifier, use of feeding tubes, use of proton-pump inhibitors, or H2 receptor antagonists), risk factors for BSIs (use of any type of venous or arterial catheter, parenteral nutrition), risk factors for catheter-associated urinary tract infection (use of urinary catheter), and risk factors for Clostridioides difficile infection (previous use of antibiotics, proton-pump inhibitors, and H2 receptor antagonists). Absolute and relative frequencies were used to summarize data.
Two time periods were compared: before HSCR (January 2019-February 2020) and during HSCR (April-July 2020). March 2020 was left out due to active HSCR. Gradual resumption of routine hospital activities began in July 2020.
Statistical Analysis
Statistical analysis by the Chi-square test or Fisher's exact test (for categorical variables) or the Wilcoxon rank-sum test (for numerical variables) was done to contrast differences between the two time periods, considering a p cutoff value of ≤0.05 as statistically significant. Stata© version 14.0 (StataCorp, College Station, TX, USA) was used.
RESULTS
Before HSCR, 449 HAIs in 72,832 patient-days were identified (HAI rate = 6.2/per 1000 patient-days). During HSCR, 170 HAIs in 14,372 patient-days occurred (HAI rate = 11.8 cases per 1000 patient-days). The increase in the total HAI rate during HSCR was due to increases in VAP and BSI rates (Fig. 1).
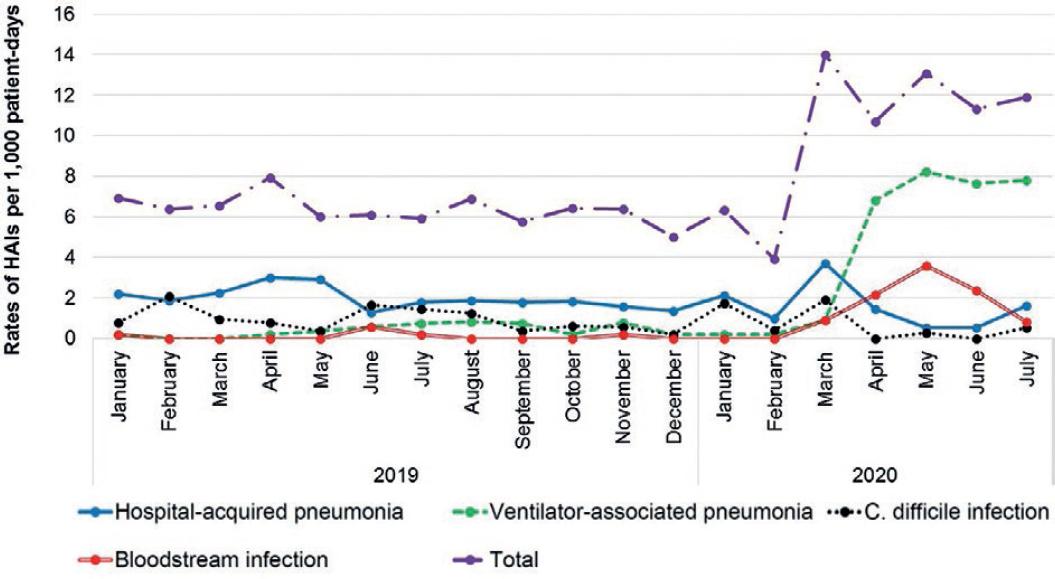
Figure 1 Healthcare-associated infection rates stratified by type of infection. Catheter-associated urinary tract infection and surgical site infection were left out for clarity; no changes were noted in rates of the former and no surgeries related to non-COVID-19 conditions were done during hospital surge capacity response.
A greater proportion of critically ill patients was admitted during HSCR, leading to an increase in the prevalence of risk factors related to insertion and use of invasive devices (Table 1).
Table 1 Comparison of patient characteristics, risk factors, and microorganisms isolated from healthcare-associated infections before and during HSCR
| Variable | Before HSCR n (%)a | During HSCR n (%)a | p |
|---|---|---|---|
| HAI rate (×1000 patient-days) | 6.2 | 11.8 | 0.023 |
| Male sex | 213 (47.4) | 117 (68.8) | <0.001 |
| Age, years (median, IQR) | 53 (41-68) | 53 (43-60) | 0.150 |
| LoS before HAI, days (median, IQR) | 13 (7-23) | 10.5 (8-17) | 0.010 |
| In-hospital death | 24 (5.3) | 55 (32.4) | <0.001 |
| Types of HAIs | |||
| VAP | 45 (10.0) | 93 (54.7) | <0.001 |
| Hospital-acquired pneumonia | 121 (26.9) | 31 (18.2) | 0.025 |
| BSI | 6 (1.3) | 35 (20.6) | <0.001 |
| Candidemia | 0 (0) | 14 (8.2) | <0.001 |
| Catheter-associated urinary tract infection | 37 (8.3) | 6 (3.5) | 0.039 |
| Surgical site infection | 113 (25.2) | 0 (0)b | NA |
| C. difficile infection | 68 (15.2) | 3 (1.8) | <0.001 |
| Other | 59 (13.1) | 2 (1.2) | <0.001 |
| Risk factors for various HAIs | |||
| Arterial catheter | 2 (0.4) | 52 (30.6) | <0.001 |
| Hemodialysis catheter | 11 (2.4) | 12 (7.1) | 0.007 |
| Central venous catheter | 306 (68.2) | 158 (92.9) | <0.001 |
| Venous peripheral catheter | 232 (51.7) | 86 (50.6) | 0.806 |
| Parenteral nutrition | 3 (0.7) | 3 (1.8) | 0.354 |
| Endotracheal intubation | 51 (11.4) | 154 (90.6) | <0.001 |
| Time from endotracheal intubation to VAP, days (median, IQR) | 9 (6-14) | 10.5 (8-16) | 0.087 |
| Use of humidifier | 19 (4.2) | 5 (2.9) | 0.458 |
| Tracheostomy | 11 (2.4) | 5 (2.9) | 0.778 |
| Nasogastric tube | 5 (1.1) | 4 (2.4) | 0.268 |
| Nasojejunal tube | 0 (0) | 27 (15.9) | <0.001 |
| Use of PPI or H2 receptor antagonists | 311 (69.3) | 116 (68.2) | 0.806 |
| Urinary catheter | 186 (41.4) | 155 (91.2) | <0.001 |
| Use of antibiotics before HAI | 381 (84.9) | 121 (71.2) | <0.001 |
| Hospital area where HAI was identified | |||
| Wards | 365 (81.3) | 9 (5.3) | <0.001 |
| Emergency department | 44 (9.8) | 108 (63.5) | |
| ICU | 39 (8.7) | 38 (22.4) | |
| Accessory critical area | NA | 15 (8.8) | |
| Other area | 1 (0.2) | 0 (0) | |
| Hospital area where HAI was identified | |||
| Microorganisms isolated | 288 | 207 | |
| Gram-negative bacilli | 140 (48.6) | 141 (68.1) | <0.001 |
| Enterobacter cloacae | 4 | 33 | |
| Escherichia coli | 34 | 22 | |
| Klebsiella pneumoniae | 16 | 18 | |
| Other Enterobacteriaceae | 28 | 28 | |
| Pseudomonas aeruginosa | 27 | 24 | |
| Other non-fermenting Gram-negative bacilli |
31 | 16 | |
| Gram-positive cocci | 42 (14.6) | 27 (13.0) | 0.624 |
| Enterococci | 24 | 5 | |
| Staphylococcus aureus | 9 | 9 | |
| Coagulase-negative staphylococci | 1 | 9 | |
| Other cocci | 8 | 4 | |
| Fungi | 23 (8.0) | 35 (17.0) | 0.002 |
| Candida albicans | 9 | 9 | |
| Non-albicans Candida spp. | 6 | 14 | |
| Aspergillus spp. | 5 | 9 | |
| Other fungi | 3 | 3 | |
| C. difficile | 68 (23.6) | 3 (1.4) | <0.001 |
| Other microorganism | 15 (5.2) | 1 (0.5) | 0.003 |
| Resistant microorganisms of epidemiological importance | |||
| ESBL producers | 4/8 (50.0) | 12/29 (41.4) | 0.705 |
| AmpC producers | 6/14 (42.9) | 52/82 (63.4) | 0.146 |
| Carbapenem-resistant Enterobacteriaceae | 2/12 (16.7) | 9/79 (11.4) | 0.635 |
| Methicillin-resistant S. aureus | 2/6 (33.3) | 0/5 (0) | 0.455 |
| Multidrug-resistant P. aeruginosa | 5/7 (71.4) | 2/21 (9.5) | 0.004 |
| Carbapenem-resistant Acinetobacter baumannii |
4/4 (100) | | NA |
| Azole-resistant Candida species | | 8/13 (61.5) | NA |
aUnless otherwise stated.
bNon-COVID-19 surgical procedures were halted during HSCR.
HSCR: hospital surge capacity response; HAI: healthcare-associated infection; IQR: interquartile range; LoS: length of stay; VAP: ventilator-associated pneumonia; BSI: bloodstream infection; PPI: proton-pump inhibitor; NA: not applicable; ESBL: extended-spectrum beta-lactamase; AmpC: ampC beta-lactamase. ICU: intensive care unit, C. difficile: Clostridioides difficile.
Due to the workflow modifications during HSCR mentioned above, surveillance and estimation of adherence to the various measures of the IPC program were non-existent during HSCR.
The types of microorganisms causing HAIs differed between the two periods (Table 1). During HSCR, an increase in the proportion of Gram-negative bacilli and fungi was noted; in contrast, cases of C. difficile infection diminished.
DISCUSSION
An increase in BSI and VAP rates in our hospital was temporally associated with the interruption of our IPC program and changes in work standards due to HSCR.
Our findings are in agreement with another study that reported a notorious increase in the BSI rate in COVID-19 patients, mostly due to Gram-negative bacteria7; among the causes, a higher number of critical patients admitted to strained ICUs, reduced staff-to-patient ratios, and increased length of stay of patients were found. As noticed in that report, we also observed a reduction in our BSI rate after adoption of preventive actions. Although we are not able to provide a statistical value to this observation due to the limited HSCR time period during which BSI rates increased, there was a notorious reduction after the IPC program was resumed.
Regarding the VAP rate, our findings were similar to those of another study8: VAP in COVID-19 patients was a very frequent occurrence when mechanical ventilation was required. The rise in the endotracheal intubation rate related to the pandemic was a contributory factor5; however, prone positioning, use of muscle relaxants, a decreased rate of daily awakening protocols, and decreased surveillance of HAIs could also be associated. Of the potential associated factors mentioned previously, only resumption of surveillance was possible since modification of the others was unfortunately restricted to patients without severe disease. This could explain the lack of reduction in VAP rates.
The rates of hospital-acquired pneumonia in wards remained stable, possibly due to the admission of non-intubated and less severely ill patients in such areas.
Finally, two circumstances that may account for reduced rates of C. difficile infection were (1) not admitting patients with active infection and (2) increased adherence of hand hygiene and contact precautions by staff9.
Pending further studies, the present one suggests that HAI preventive tasks may be compromised during the COVID-19 pandemic. A previous survey found that the majority of the personnel dedicated to IPC duties spent most of the time responding to the COVID-19 emergency, undermining important activities such as surveillance of adherence to hand hygiene, chlorhexidine bathing, antimicrobial stewardship programs, and provider feedback10.
We acknowledge limitations. Because interruption of the IPC program coincided with changes in workflow and in medical procedures (especially prone positioning), we were not able to estimate the relative contribution of each separate event to the increase in HAIs during HSCR. However, the decrease in BSI rates after resumption of the IPC program in May 2020 (and accompanying additional measures specific to the prevention of BSIs) plus no further changes in the other likely associated factors previously mentioned, support the observation that interruption of the IPC program was very possibly related to the surge in HAIs. Although the use of dexamethasone could be theoretically related to increases in HAI rates elsewhere, we consider this highly unlikely in our setting since dexamethasone use was established after June 30, 2020; therefore, this variable was not analyzed in our study.
In summary, an increase in the incidence of VAPs and BSIs was noted during HSCR in a COVID-19 setting. Resumption of prevention activities allowed a reduction of BSI rates. IPC programs must remain an essential activity during the COVID-19 pandemic.











 nueva página del texto (beta)
nueva página del texto (beta)


