INTRODUCTION
For the treatment of severe COVID-19 patients, several therapeutic procedures have been suggested, with controversial results, although no attention has been paid to pharmacological intervention for the treatment of hypoxemia and respiratory distress. In severe cases, patients continue to have increased respiratory distress and hypoxemia despite a high percentage of oxygen therapy. For alleviating hypoxemia and respiratory distress, all attention have been focused on using only oxygen support by non-invasive or invasive ventilation1,2.
In our first trial, we explained in detail the possible pathogenesis of COVID-19 and the safety of methylene blue (MB) in the treatment of COVID-19 patients3. There are two forms of MB, oxidized and reduced. The oxidized form is an oxidant that exacerbates oxidative stress; contrary, the reduced form (Leukomethylene [LMB]) is an antioxidant that alleviates oxidative stress. LMB (reduced form) decreases hypoxemia through its antioxidant effect, resulting in alleviating respiratory distress3-5. This trial was designed to evaluate the efficacy of MB (the reduced form) for treating severe hospitalized COVID-19 patients by correcting hypoxemia and respiratory distress.
METHODS
Study subjects
The study was performed at three hospitals of Mashhad University of Medical Sciences, Mashhad, Iran, after ethics committee approval (IR.MUMS.REC.1399.122; ClinicalTrials.gov Identifier: NCT04370288; April 19, 2020) and taking written informed consent from patients. Enrollment for the clinical trial began on June 22, 2020, and ended on August 22, 2020. The authors were responsible for designing the trial and for collecting and analyzing the data. The clinical trial has been conducted according to the principles expressed in the Declaration of Helsinki.
Study design
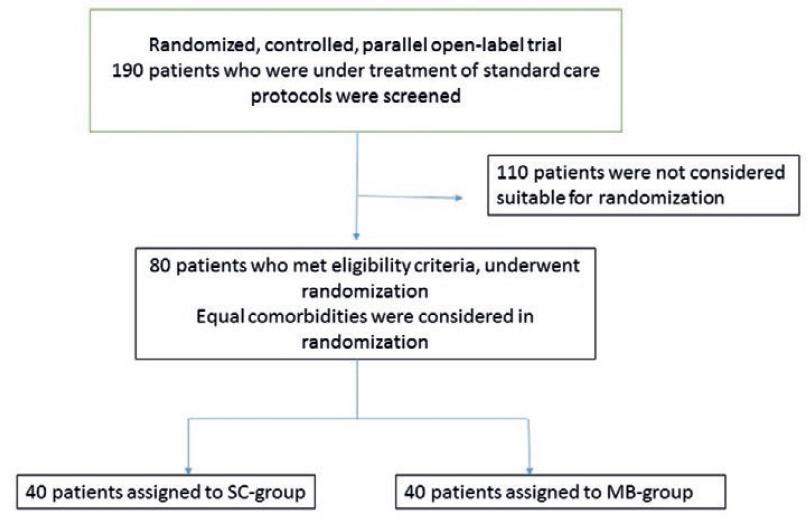
This study was a randomized, controlled, parallel, open-label trial. Neither statistician nor investigators or patients were masked to the treatment assignment (Fig. 1). No drugs were masked, and a placebo was not used. Inclusion criteria were severe patients with age above 18 years old, respiratory distress (≥26 breaths/min), oxygen saturation ≤93% at rest in the room (FiO2 = 21), and a confirmed case of COVID-19 (by reverse transcription polymerase chain reaction on the nasopharyngeal swab collected or clinical and typical high-resolution computed tomography features), which had no sign of improvement after 5 days of the standard of care (SOC) treatment. Exclusion criteria were a history of G6PD deficiency, severe renal failure, body mass index more than 30 kg/m2, cirrhosis, active chronic hepatitis, a history of an allergic reaction to MB, treatment with immunosuppressive agents, pregnancy, breastfeeding, and the presence of any condition that would not allow the protocol to be followed safely, such as cognitive impairments or poor mental status.
To achieve the sample size of 80, 190 hospitalized patients were screened (Fig. 1). To decrease the effect of confounding factors, cluster randomization was performed to equalize comorbidities in each group. Eligible patients were randomly included and stratified by their pre-existing conditions in a 1:1 ratio to either the MB group (40 patients) or the SOC group (40 patients).
METHODS
MB syrup formulation
The syrup contained MB, Vitamin C, dextrose, and N-acetyl cysteine. The special formulation for MB (the reduced form) was patented (IR-139950140003002083) (on June 1, 2020, PCT). The syrup was made by dissolving MB (USP) (14 mg/mL) in a simple syrup (50% sucrose). The electrochemical reduction process was performed in the presence of dextrose (500 mg/mL, at 70°C, 40 min), Vitamin C (140 mg/mL at 30°C, 50 min), and N-acetyl cysteine (150 mg/mL at 30°C, 50 min). In this study, the conversion index of MB to LMB was almost zero absorption in the wavelength of 660 nm, when the syrup was diluted to a concentration of 4 mg/L in distilled water. Accelerated stability studies (40°C ± 2°C) were done for a period of 3 months and no significant changes were observed during this time. However, all drugs were used within 3 months.
Intervention
In the MB group, along with SOC, MB syrup was administered orally to patients (1 mg/kg every 8 h for 2 days, followed by 1 mg/kg every 12 h for the following 12 days). In the SOC group, SOC protocol was continued. SOC protocols were applied according to the WHO guidelines. In SOC protocols, severely ill patients receive supplemental oxygen, intravenous fluids, antiviral agents, antibiotics, anticoagulants, and corticosteroids6,7.
During MB therapy, patients were assessed on each visit for oxygen saturation (SpO2) and respiratory rate (RR, number of breaths per minute) at rest in the room air (FiO2 = 21) after lunch. The primary outcomes were SpO2 level and RR on the 3rd and 5th days. The secondary outcomes were hospital stay and mortality rate within 28 days. It should be noted that hospital stay was counted from the day after MB treatment. Decrease in RR was considered as improvement of respiratory distress.
Statistical analysis
Continuous variables were compared by the MannWhitney U-test. Furthermore, Chi-square and Fishers exact tests were used for categorical variables. The mean difference of SpO2 and RR was calculated for each study group. The Wilcoxon rank sign test was used to compare the mean difference of these variables for each study group. The significance level was <0.05 in all statistical analyses. SPSS version 23 was used in statistical analysis. The rate ratio calculation for SpO2 (or RR) on the 3rd day was the mean difference of SpO2 (or RR) on the 3rd day in the MB group divided by the mean difference of SpO2 (or RR) on the 3rd day in the SOC group. The rate ratio calculation for SpO2 (or RR) on the 5th day was the mean difference of SpO2 (or RR) on the 5th day in the MB group divided by the mean difference of SpO2 (or RR) on the 5th day in the SOC group.
Role of the funding source
The funders did not have any role on the design, collection, management, analysis, interpretation of data, writing of the report, or the decision to submit the report for publication. This work was supported by a grant from Mashhad University of Medical Sciences (Grant number: 990096).
Ethics approval statement IR.MUMS.REC.1399.122; Clinical Trials.gov Identifier: NCT04370288; April 19, 2020.
RESULTS
Patients
Demographic characteristics of patients in the MB and SOC groups are presented in Table 1. There were no significant differences in demographic characteristics between both groups.
Table 1 Baseline patient characteristics of methylene plus standard of care group (MBG) and SOC group (SCG)
| Patients | MBG (n = 40) | SCG (n = 40) | SD | |
|---|---|---|---|---|
| Age | 53/7 ± 13/ | 55/2 ± 13/8 | n, p = 0.8 | |
| Male/female | 19/21 | 23/17 | n, p = 0.3 | |
| Antivirals (hydroxychloroquine + Kaletra) (5 days) | 100 | 100 | ||
| Antibiotic | ||||
| Ceftriaxone (7 days) | 70% | 75% | n, p = 0.9 | |
| Azithromycin (5 days) | 95% | 90% | ||
| Meropenem (7-10 days) | 30% | 25% | ||
| Vancomycin (7-10 days) | 30% | 25% | ||
| Anticoagulant (up to discharge) | ||||
| Prophylactic | 85% | 90% | n, p = 0.9 | |
| Therapeutic | 10% | 5% | ||
| No anticoagulant | ||||
| Immunosuppressant and Immunomodulatory agents | 5% | 5% | n, p = 0.9 | |
| Dexamethasone (10 days) | 90% | 85% | ||
| Atorvastatin (up to discharge) | 95% | 90% | ||
| Interferon beta (3-5 days) | 30% | 35% | ||
| Comorbidities | ||||
| No medical history | 16 | 18 | n, p = 0.9 | |
| Hypertension | 7 | 7 | ||
| Diabetes | 3 | 5 | ||
| Diabetes + Hypertension | 7 | 7 | ||
| Others | 5* | 5# | ||
*One patient: Downs syndrome; one patient: rheumatoid arthritis; one patient: Gout disease; one patient: coronary artery bypass graft; one patient: hypothyroidism, kidney stone, ischemic heart disease.
#One patient: coronary artery bypass graft, one patient: breast cancer; one patient: lymphoma; one patient: prostate cancer; one patient: ischemic heart disease. SD: significant difference; SOC: standard of care.
Primary outcomes
In the MB group, patients had a significant increase of SpO2 on the 3rd day (mean difference [MD]: 5.4; 95% confidence interval [CI]: 3.4-7.4; p < 0.0001) and on the 5th day (MD: 8.9, 95% CI: 5.5-12.2; p < 0.0001). In the SOC group, there was no significant increase in SpO2 on the 3rd day (MD: 0.4, 95% CI: −0.3-1.294; p = 0.24); however, patients had a significant increase in SpO2 on the 5th day (MD: 4.3, 95% CI: 1.8-6.9; p = 0.001) (Table 2).
Table 2 Changes of oxygen saturation (SpO2) and RR in MBG and SCG groups
| SpO2 | Before MB | 3rd day fter MB | 5th day after MB | RR | Before MB | 3rd day after MB | 5th day after MB |
|---|---|---|---|---|---|---|---|
| MBG | 80.0 ± 9.3 | 85.4 ± 7.8 a: y p < 0/0001 | 88.9±9.8 b: y p < 0/0001 | MBG | 34.4±5.5 | 25.3 ± 4/4 a: y p < 0/0001 | 22.7 ± 6.7 b: y p < 0/0001 |
| SCG | 79.8 ± 7.5C | 80.2 ± 7.4 a: n p = 0.24 | 84.1 ± 9.7 b: y p = 0.002 | SC-G | 32.0 ± 4.8d | 31.1 ± 4.3 a: n p = 0.20 | 28.8 ± 5.8 b: y p = 0.01 |
Data are presented as mean ± SD (standard deviation).
aSignificant difference (SD) between the 3rd day after and before MB therapy.
bSignificant difference (SD) between the 5th day after and before MB therapy.
y: yes; n: no; there was no significant difference of SpO2 (c: p = 0.461) and RR (d: p = 0.1) between MBG and SCG before MB therapy. RR: respiratory rate.
In the MB group, patients had a significant decrease of RR on the 3rd day (MD: −9.1, 95% CI: −11.0-−7.1; p < 0.0001) and on the 5th day (MD: −11.6, 95% CI: −14.7-−8.5; p < 0.0001). In the SOC group, patients had no significant decrease of RR on the 3rd day (MD: −0.9, 95% CI: −2.6-0.6; p = 0.20), but there was a significant decrease of RR on the 5th day (MD: −3.1, 95% CI: −5.4-−0.8; p = 0.01) (Table 2).
The mean differences of SpO2 and RR changes were higher in the MB group compared with the SOC group on the 3rd and 5th days (Table 3 and Fig. 2). In the MB group in comparison to the SOC group, the rate ratio of increased SpO2 was 13.5 and 2.1 times on the 3rd and 5th days, respectively. In the MB group in comparison to the SOC group, the rate ratio of RR improvement was 10.1 and 3.7 times on the 3rd and 5th days, respectively.
Table 3 Comparison of oxygen saturation (SpO2) and RR between MBG and SCG groups
| SpO2 & RR | MBG | SCG | p* |
|---|---|---|---|
| Median SpO2 baseline | 83.5 (73.5-88) | 82 (74.2-85) | 0.46 |
| Median SpO2 on 3rd days after intervention | 89 (83.25-90.75) | 82 (76.25-86) | <0.001 |
| Median SpO2 on 5 days after intervention | 93 (88-95) | 87 (80.25-91.75) | 0.01 |
| Mean difference of SpO2 after 3 days | 4 (2-8.75) | 1 (-2-2.75) | <0.001 |
| Mean difference of SpO2 after 5 days | 7 (6-14) | 6 (1-10) | 0.05 |
| Median respiratory rate baseline | 35.5 (29.2-39) | 31.5 (28.2-35) | 0.07 |
| Median respiratory rate on the 3rd days after intervention | 25 (21-28.7) | 30.5 (28-34.7) | <0.001 |
| Median respiratory rate on 5 days after intervention | 21 (18.2-23) | 28.5 (24-33.75) | <0.001 |
| Mean difference of respiratory rate after 3 days | −10 (−12-−5) | −3 (−4-3) | <0.001 |
| Mean difference of respiratory rate after 5 days | −13.5 (−17.7-−9) | −4 (−8-2) | <0.001 |
Data are presented by median (interquartile range).
*MannWhitney U-test.
RR: respiratory rate.
Secondary outcomes
After MB therapy, the hospital stay was significantly shortened in the MB group compared with the SOC group (MD: −3.8, 95% CI: −6.3-−1.2; p = 0.004). The mortality rate in the MB and SOC groups was 12.5% and 22.5%, respectively (Table 4). The change in mortality rate was not significant (MD: −0.10, 95% CI: −0.27-0.06; p = 0.24), but it was reduced by 10%. No serious adverse effects were observed in the MB group except for the color of the patients urine, which turned to green or blue.
Table 4 Hospital stay and mortality rate in MBG and SCG groups
| Patients | MBG (n = 40) | SCG (n = 40) | SD |
|---|---|---|---|
| Hospital stay* (days) | 7.3 ± 4.7 | 11.7 ± 6.6 | y p = 0/004 |
| Day 28th: mortality n (%) | 5 (12.5%) | 9 (22.5%) | n p = 0.24 |
*Patients were under treatment for 5 days and did not improve, and then, MB therapy started. The hospital stay was counted from the day after MB treatment.
N: number of dead patients; SD: significant difference; y: yes; n: no.
Side effects of MB
The side effects of MB were one patient with a very light headache that resolved after 10 min; and one patient who vomited after using MB, and then did not consent to take part in the trial. Confusion, increase in blood pressure, and shortness of breath were not seen among the patients. These findings may be related to the fact that reduced MB was used instead of oxidized MB; further research could clarify this matter. To rule out toxic effects of MB, blood count, liver enzymes, and kidney function tests at the start and the end of MB therapy were compared (Table 5).
Table 5 The blood count, liver enzymes, and kidney function tests at the beginning and at the end of MB therapy
| Test | Before MBT | After MBT | Significant difference p-value |
|---|---|---|---|
| Urea | 39.0 ± 17.9 | 44.5 ± 14.4 | No, 0.2 |
| Creatinine | 0.93 ± 0.17 | 0.86 ± 0.19 | No, 0.16 |
| ALT | 58.1 ± 105.5 | 72.4 ± 78.5 | No, 0.59 |
| AST | 67.4 ± 103.8 | 55.3 ± 59.1 | No, 0.60 |
| WBC | 8.2 ± 4.1 | 8.6 ± 4.5 | No, 0.77 |
| PMN | 81.2 ± 9.7 | 80.5 ± 6.5 | No, 0.74 |
| Lymphocyte | 12.1 ± 7.1 | 13.3 ± 6.2 | No, 0.51 |
MBT: methylene blue therapy; WBC: white blood cell; PMN: polymorphonuclear; ALT: alanine aminotransferase; AST: aspartate transaminase. MB: methylene blue.
DISCUSSION
This trial showed that MB, as a supplementary therapy to SOC protocols, led to a significant increase in SpO2, a significant decrease of respiratory distress and hospital stay, and 10% decrease in mortality rate. Severe COVID-19 patients presented with the chief complaint of dyspnea. After 1 day of MB administration, 92% of patients expressed dyspnea relief. This finding was very important for the care of COVID-19 patients suffering from respiratory distress.
In the MB group, the history of patients who had died highlighted that the best time for MB intervention was at the early stages of hypoxemia before requiring mechanical ventilation. The change in mortality rate was not significant (although there was a decrease of 10%), which may have been due to the small number of patients in this study.
In our previous trial, we discussed one of the possible biochemical processes which may be involved in the pathogenesis of the disease. It is the activation of macrophages by viruses that produce a huge amount of nitric oxide (NO). NO takes part in producing the highly reactive oxygen species (ROS) and also is converted to nitrite in blood by ceruloplasmin. ROS and nitrite pass easily through the red blood cell membrane and oxidize ferrous to ferric. Oxygen cannot attach to ferric ion in hemoglobin (methemoglobin) which results in hypoxemia3.
The rationale for considering MB for treatment was the following proven mechanisms: (1) MB has antiviral activity against COVID-19 by inhibiting in vitro the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spikeACE2 protein-protein interaction8. MB can prevent the cytopathic effect and reduce the propagation of RNA virus9. (2) MB is an FDA-approved drug in the treatment of methemoglobinemia10. (3) MB has direct inhibitory effects on NO synthases (produces NO that takes part in generating reactive nitrogen species, which damage the cells and biomolecules) and guanylate cyclase enzyme11. (4) MB increases the activity of normally slow NADPHmethemoglobin reductase pathway, which decreases hypoxemia through reducing methemoglobin12. (5) MB has formed the basis of antimicrobial chemotherapy, particularly in the area of antimalarials. It is used in an antibacterial foam dressing for the management of chronic wounds with local infection13. (6) MB is a powerful oxygen superoxide scavenger that eliminates rapidly this ion to avoid damage to tissue14. (7) MB inhibits xanthine oxidase, which prevents ROS production15. (8) MB prevents platelet activation, adhesion, and aggregation16. (9) MB (the reduced form) quenches ROS as a reducing agent17. (10) MB (the reduced form) decreases inflammation18.
In this study, after the administration of MB (the reduced form, colorless), the color of urine and feces of patients turned to green or blue. Patients whose urine or feces had the green color, recovered (35 patients), but five patients who had dark blue color in urine or feces died. In our previous trial, we demonstrated high oxidative stress in COVID-19 patients3. When MB (oxidized form, dark blue) is orally administered, by oxidizing other antioxidants, it is converted to the reduced form (colorless)19, which is excreted primarily in the urine20. Therefore, the oxidized form of MB exacerbates the oxidative stress in COVID-19 patients, worsening hypoxemia. However, the reduced form of MB, as an antioxidant, quenches the oxidative stress and also decreases hypoxemia by converting the ferric to the ferrous ion in hemoglobin. In this trial, after the administration of MB (the reduced form), since there were a large number of oxidants in patients3, they oxidized the reduced form of MB (LMB) and turned it to the oxidized form, which was excreted in the urine in blue color. Dark blue in the urine reflected high oxidative stress in patients. This phenomenon could be considered as a prognostic factor; patients whose urine turns to a dark blue color usually have a worse outcome which requires more advanced intervention. These patients may need a cocktail of antioxidants along with the reduced form of MB.
Limitations of the study include that the trial was conducted in one university center with a small number of patients.
MB therapy along with SOC may be efficacious in the treatment of COVID-19. This supplementary treatment may improve patient outcomes (increasing SpO2 and decreasing respiratory distress, hospital stay, and mortality rate) without serious adverse effects. MB is an FDA-approved drug for methemoglobinemia. Since MB is inexpensive and ubiquitously accessible, this drug may be used as a supplementary choice for the treatment of hypoxemia in COVID-19 patients. We suggest that the ideal time for MB administration should be on diagnosis and at least before the severe stage of the disease and multiorgan involvement and failure. MB may also be used for prevention, since it can protect the population by inhibiting the SARS-CoV-2 spikeACE2 interaction8, and can also reduce the propagation of RNA virus9. If the findings of this trial are verified by larger clinical trials and other research centers, it could save COVID-19 patients from stressful respiratory distress and can reduce hospital stay and mortality.











 nueva página del texto (beta)
nueva página del texto (beta)