INTRODUCTION
The prevalence of restrictive spirometric pattern has been described by several population-based surveys, one of the mayor findings is that this group has a decreased survival1,2 just as important as that observed in moderate airflow obstruction and worse than mild chronic obstructive pulmonary disease (COPD)3-6, with an adjusted hazard ratio as high as 2.86. Individuals with a restrictive pattern more often have respiratory symptoms, obesity, diabetes, metabolic syndrome, and cardiovascular morbidity7-9.
In the COPD gene study, the restrictive pattern (preserved ratio impaired spirometry, low forced expiratory volume in 1 s [FEV1] with normal FEV1/forced vital capacity [FVC] ratio)5 was considered a transitional stage, as 47% of individuals changed into another spirometric pattern, 22% into a normal spirometry, and 25% into an obstructive pattern, which may be true in smokers but not necessarily in never-smokers.
COPD is an under-diagnosed and under-treated condition, and one of the main reasons for this is poor access to spirometers10. Peak expiratory flow (PEF) can be employed to detect severe airflow obstruction in smokers over age 4011,12. A low PEF (<80 percentage of predicted [%P]) allows to prescribe diagnostic quality post-BD spirometry only to 12% of the screened population11. A similar strategy has been tested with other low-cost spirometers in selected populations13,14, several of these utilizing the advantages of a 6 s spirometry, a simplified alternative to performing a prolonged expiration15-23, based on FEV1/FEV6 which is nearly equivalent to, simpler, and possibly more specific than standard criteria based on FEV1/FVC20.
In the Proyecto Latinoamericano de Investigación en Obstrucción Pulmonar (PLATINO) cohort, the FEV1, currently measurable with inexpensive pocket spirometers, was the main predictor of survival24 as well as a predictor of lung function decline25. In Mexico City, we found a 6 s spirometer, helpful to optimize the selection of individuals for diagnostic-quality pre- and post-BD spirometry for airflow obstruction based on a crude FEV1/FEV6 of <0.826. For the purposes of this study, the objective was to evaluate the performance of 6s spirometry as a screening tool for all spirometric ventilatory abnormalities, including the restrictive pattern, utilizing the FEV1, FEV6, and their quotient, crude and expressed as the %P.
METHODS
Study design
This study consisted of multistage population-based cluster sample of Mexico City Metropolitan area residents, conducted in 2010, designed for the PLATINO study, and performed 7 years earlier; the methods have been described in detail27,28. Eligible were all non-institutionalized residents aged 40 and older, the majority of whom had also participated in the previous survey. Interviewers applied the PLATINO questionnaire, available online29. Our Institutions Ethics Committee approved the study (E04-10), and all participants provided a signed informed consent.
Spirometry was performed by trained technicians at the participants home, using portable, spirometers (NDD Medical Technologies, Zurich, Switzerland), utilizing a flow volume transit time ultrasonic sensor, before (pre-BD) and 15 min after the administration of 200 μg of salbutamol (post-BD) following the American Thoracic Society/European Respiratory Society (ATS/ERS) procedures30. Participants also performed three, 6 s maximal expiratory maneuvers with a turbine-based, simplified, pocket spirometer (COPD-6 model 4000, Vitalograph Ltd., Ennis, Ireland). The order of the tests was randomly allocated. The highest FEV1 and FEV6 values, and their quotient, from the three measurements were employed for analysis. Measurements were expressed as crude values and as the %P by reference values for Mexican-American population31.
Restriction was defined as a post-BD FEV1 or FVC< lower limit of normal (LLN) of reference equations for Mexican-Americans31, without airflow obstruction (FEV1/FVC≥LLN). Severity of spirometric abnormalities (obstructive and restrictive) was classified as proposed by ATS/ERS32, based on FEV1%P as follows: FEV1%P ≥ 70<LLN= mild, 60≤ FEV1%P <70= moderate, 50≤FEV1%P <60= moderately severe, and FEV1%P <50= severe.
Statistical Analysis
Non-parametric receiver operating characteristic (ROC) curves were utilized to assess the discriminatory power of the pre-bronchodilator results from the COPD-6 (FEV1, FEV6, and FEV1/FEV6) crude and as %P (with adjusted differences due to gender, body size, and age)31, to identify appropriate cutoff values to estimate pre- and post-bronchodilator spirometric abnormalities. The best cutoff points were selected by the highest Youden index (the Youdens J statistic): J= sensitivity+ specificity -1, conferring the same weight on false positives and false negatives.
Estimates of the proportion of the total screened population requiring confirmatory spirometry and of its combined positive predictive value (PPV) were obtained for a range of prevalence values employing the Bayes theorem. All analyses were performed using STATA statistical software33.
RESULTS
The type of spirometer used or the order in which the 6 s spirometry and diagnostic-quality spirometry did not affect the results, and those factors were not further considered in the analysis. In the 2010 Mexico City survey, we identified 1040 eligible subjects. Pre-BD spirometry with complete questionnaires and 6 s spirometry tests were available for 742 individuals; 670 of them were also submitted to a post-BD spirometry.
Summary measures for relevant variables are presented in Table 1 for the analyzed sample. About 40% of participants were men and respiratory symptoms had a low prevalence. Furthermore, low was the prevalence of medically diagnosed asthma (4.6%), COPD (0.7%), and previous tuberculosis (<1%). The majority of individuals were never-smokers (57%), and only 16% reported >10 pack-years. Exposure to a dusty job for more than 1 year was reported by 39%, and cooking with a biomass stove, by 6%.
Table 1 Characteristics of participants with pre-BD spirometry
| Demographic | 2010 Mexico city survey sample (n = 742) |
|---|---|
| Age group (years) % | |
| 40-49 | 29.1 |
| 50-59 | 30.8 |
| 60 and older | 40.1 |
| % men | 40.3 |
| Anthropometry | |
| Height (cm, Mean [SD]) (cm, mean [SD]) | 156.5 (9.4) (9.1) |
| Weight (kg, Mean [SD]) (Kg, mean [SD]) | 71.2 (14.0) (13.9) |
| Body mass index (kg/m2, (Mean [SD]) (Kg/m2, mean [SD]) | 29.1 (5.1) (5.1) |
| % Obese | 36.0 |
| Respiratory symptoms, % with | |
| Cough and phlegm on most days for at least 3 months per year | 4.5 |
| An attack of wheezing with shortness of breath in the past 12 months | 3.0 |
| Percent with previous medical diagnosis of | |
| Asthma | 4.6 |
| COPD | 0.7 |
| Tuberculosis | 0.8 |
| Exposures | |
| Pack-years of cigarette smoking, % | |
| Never-smoker | 56.6 |
| 1-9 | 27.7 |
| 10 and more | 15.7 |
| % who worked at a dusty job for more than 1 year | 39.6 |
| Years exposed to wood smoke from cooking, mean (SD) (Mean, [SD]) | 6.2 (9.5) (8.7) |
| Pre-bronchodilator spirometric abnormalities (n = 742) | |
| (FVC<LLN OR FEV1 <LLN) and FEV1/FVC≥LLN (restrictive), %, (n) | 9.7 (72) |
| FEV1/FVC<LLN (Obstructive), %, (n) | 4.5 (33) |
| FVC<LLN OR FEV1 <LLN OR FEV1/FVC<LLN (any abnormality) | 14.2 (105) |
| Post-bronchodilator spirometric abnormalities (n = 670) | |
| (FVC<LLN OR FEV1 <LLN) and FEV1/FVC≥LLN (restrictive), %, (n) | 9.3 (62) |
| FEV1/FVC<LLN (obstructive), %, (n) | 2.5 (17) |
| FVC<LLN OR FEV1 <LLN OR FEV1/FVC<LLN (any abnormality) | 11.8 (79) |
LLN: lower limit of normal, the lower 5th percentile. COPD: Chronic Obstructive Pulmonary Disease; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
Intratest coefficients of variation for FEV1, FEV6, and FEV1/FEV6 from the COPD-6 device were higher than those obtained from the spirometer (Table 2). In addition, crude values of FEV1, FEV6, and FEV1/FEV6 from 6 s spirometry had a significant intraclass correlation with spirometric pre-BD values: 0.95 (95% CI 0.94-0.96) for FEV1, 0.87 (95% CI 0.86-0.89) for FEV6, and 0.34 (95% CI 0.29-0.39) for FEV1/FEV6. For measurements expressed as %P, these were 0.85 (95% CI 0.84-0.87) for FEV1%P, 0.66 (95% CI 0.62-0.9) for FEV6%P, and 0.30 (95% CI 0.25-0.35) for FEV1/FEV6%P. Concordance correlation coefficient between FEV1%P from the COPD-6 and the pre-BD spirometry was 0.85 (SE 0.01) and 0.80 (SE 0.01) with post-BD spirometry.
Table 2 Spirometry parameters (means and SD) for diagnostic-quality spirometry and 6 s spirometry (both pre-BD) (n = 742)
| Spirometry parameters | Diagnostic spirometry | 6 s spirometry |
|---|---|---|
| FEV1 (L, mean [SD]) | 2.46 (0.71) | 2.44 (0.70) |
| FEV6 (L, mean [SD]) | 3.05 (0.84) | 2.85 (0.80) |
| FEV1/FEV6 (mean [SD]) | 0.81 (5.5) | 0.86 (8.2) |
| FEV1 (% predicted, mean [SD]) | 98.5 (17.3) | 97.4 (16.5) |
| FEV6 (% predicted, mean [SD]) | 99.1 (16.2) | 92.7 (16.4) |
| FEV1/FEV6 (% predicted, mean [SD]) | 100.9 (7.9) | 105.2 (9.7) |
| Grade A test quality (%) | 79a | 70.6b |
| Repeatability for FEV1 and FEV6 Pre-BD* | 82.0 | 70.6 |
| Pre-BD intratest CV FEV1 | 2.3 (3.7) | 3.3 (6.0) |
| Pre-BD intratest CV FEV6 | 2.0 (2.8) | 3.9 (7.3) |
| Pre-BD intratest CV FEV1/FEV6 | 0.9 (1.4) | 2.8 (10.1) |
| Severity of pre-BD FEV1%P abnormalities (N and %) | ||
| Normal (≥LLN) | 678 (91.4) | 673 (90.7) |
| Mild (≥70<LLN) | 38 (5.1) | 38 (5.1) |
| Moderate (≥60 <70) | 17 (2.3) | 23 (3.1) |
| Moderate-severe(≥50<60) | 5 (0.7) | 4 (0.5) |
| Severe (<50) | 4 (0.5) | 4 (0.5) |
aFEV1 and FVC repeatable within 150 mL. CV is the coefficient of variation. Severity of FEV1 abnormality in restrictive and obstructive patterns by ATS/ERS criteria.
bThree maneuvers with the two best FEV1 and FEV6 matching within 150 mL; FEV1: forced expiratory volume in 1 s; %P: percentage of predicted; FVC: forced vital capacity; LLN: lower limit of normal.s
Spirometric abnormalities before bronchodilator use were observed in 14.2% of participants (obstructive pattern in 4.5% and a restrictive pattern in 9.7%), whereas after bronchodilator, these were observed in 11.8% (obstructive in 2.5% and restrictive in 9.3%, respectively). From the total of pre-BD spirometries, FEV1%P was normal in 678 (91.4%) and low in the remaining: reduction was mild in 38 (5.1%), moderate in 17 (2.3%), moderately severe in 5 (0.7%), and severe in 4 (0.5%). In the post-BD test, 623 (93.0%) were normal and low in the remaining: reduction was mild in 30 (4.5%), moderate in 11 (1.6%), moderately severe in 2 (0.3%), and severe in 4 (0.6%). Agreement among classification of severity between FEV1% P from COPD-6 and from pre-BD spirometry was 93.1% (kappa=0.59) and with post-BD spirometry was 92.8% (kappa = 0.52).
Figure 1 depicts the ROC curves for detecting pre-BD restrictive, obstructive, and any type of spirometric abnormality using the COPD-6, while figure 2 presents similar curves for post-BD abnormalities. For the three pre-BD abnormalities, the best predictor was pre-BD FEV1 obtained from the COPD-6, expressed as %P (FEV1%P) with an area under the curve (AUC) of 89% (for obstructive pattern), 92% (for restrictive pattern), and 91% for any abnormality. Best cutoff point was 87% P, demonstrating a sensitivity of 81-88%, and a specificity of about 84%, with a relatively low (PPV, 21% for obstructive and 47% for any abnormality), and a high negative predictive value (NPV, 97-99%) (Table S1). The results for FEV1%P were better than those for FEV6%P, even for individuals with a restrictive pattern, and then FEV1/FEV6%P for an obstructive pattern. Crude COPD-6 measurements performed considerably worse than those expressed as %P for any spirometric pattern, before or after bronchodilator (Fig.) (Table S2).
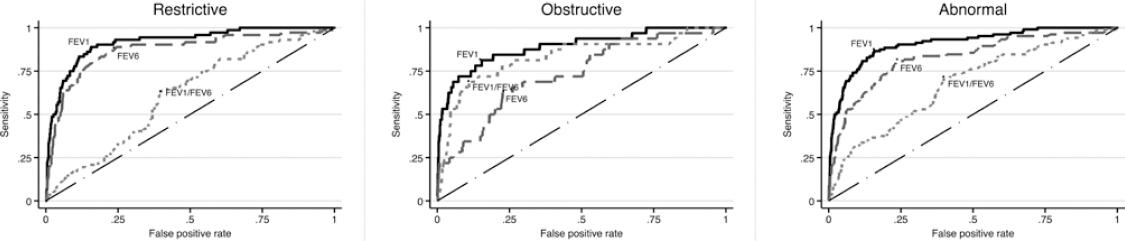
Figure 1 Receiver operating characteristics curves and area under the curve (AUC) to predict a pre-BD restrictive pattern (left graph) obstructive abnormality (middle graph) and any ventilatory abnormality (obstructive or restrictive, right graph) based on pre-BD 6 s spirometry. AUC was between 60 and 98% and highest for the FEV1 expressed as percentage of predicted (%P), the best predictor, with an AUC of 91.8 for the restrictive pattern, 89% for the obstructive pattern, and 91% for any ventilatory abnormality. For restriction forced expiratory volume in 6 s (FEV6) was near FEV1 and for obstruction, FEV1/forced vital capacity was near FEV1. AUC for crude chronic obstructive pulmonary disease-6 measurements was considerably lower than those depicted in the graphs expressed as %P.
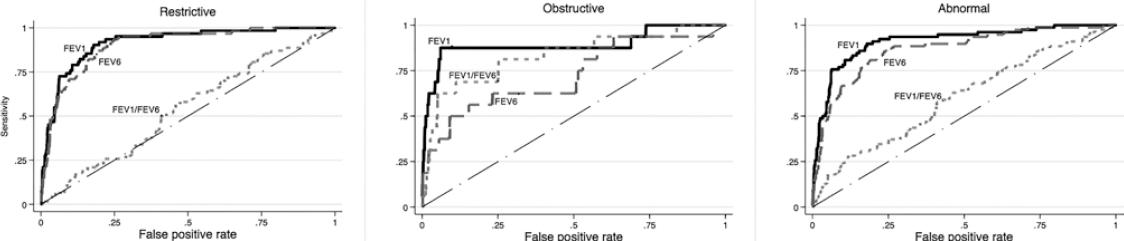
Figure 2 Receiver operating characteristics curves and area under the curve (AUC) to predict a post-BD restrictive pattern (left graph), obstructive abnormality (middle graph), and any ventilatory abnormality (obstructive or restrictive, right graph) based on pre-BD 6 s spirometry. AUC was between 54 and 91.7% and highest for the forced expiratory volume in 1 s (FEV1) percentage of predicted (%P), the best predictor, with an AUC of 91.7% for the restrictive pattern, 89% for the obstructive pattern, and 91.2% for any ventilatory abnormality. For restriction, FEV6 was near FEV1; for obstruction, FEV1/forced vital capacity was near FEV1. AUC for crude chronic obstructive pulmonary disease-6 measurements was considerably lower than those expressed as %P.
In univariate logistic regression models, COPD-6 measurements as continuous variables, expressed as %P, also had the highest proportion of spirometric pattern variation explained: for FEV1%P were 0.40, 0.35, and 0.41 for the restrictive pattern, obstructive, and any ventilatory abnormality, respectively, which were significantly higher than those for FEV6%P (0.27, 0.10, and 0.22) and then FEV1/FEV6%P (0.02, 0.17, and 0.06), which were considerably higher than those from the crude COPD-6 measurements, ranging between 0.01 and 0.05, except for the obstructive pattern (for FEV1 = 0.10 and for FEV1/FVC= 0.17). For post-BD values, the AUC was of very similar magnitude as those for pre-BD values, and the best predictor was, again, the FEV1%P (Table S1). Respiratory symptoms and pack-years of smoking, alone or in combination, had no additional value to performing a simplified spirometry to predict spirometric abnormalities, as shown before for predicting airflow obstruction with simplified spirometry26 or with PEF12. For example, AUC for >10 pack-years of smoking was of 0.51, for habitual cough or phlegm was of 0.55, for >10 pack-years of smoking or the presence of any respiratory symptom (cough, wheezing, dyspnea, or phlegm) was of 0.53, and for previous physician diagnosis of COPD, chronic bronchitis, or emphysema was of 0.54.
An FEV1 <87%P (the best cutoff point) from the COPD-6 correctly identified 14/17 (82%) of the individuals with post-BD airflow obstruction (sensitivity, specificity, PPV, and NPV in Table S1 and S2). It also identified 55 of 62 (89%) of those with restrictive pattern and 69 of 79 (87%) of those with any ventilatory abnormality. If applied as a screening test, using the 87%P cutoff point, only 194 of the 741 participants (26%) would be referred to diagnostic spirometry with very few false negatives, the majority of these mild abnormalities: among nine false negatives of post-BD spirometric abnormalities, only four had a FEV1 or a FVC<80%P, and none had a value of<60%P.
Figure 3 summarizes performance parameters (PPV and percentage of individuals below the cutoff point, FEV1%P<87, in whom a referral for diagnostic spirometry is indicated) with a range of prevalence of spirometric abnormality values, taking into account the COPD-6 exclusively. For example, with a prevalence of 15%, as found in our survey, only about 25% of the screened individuals would be sent for spirometry, with a PPV near 0.5, whereas for a prevalence of 30%, closer to what can be expected in an outpatient clinic, about 35% would be sent for spirometry with a PPV of 0.7, in both situations with a NPV >0.97.
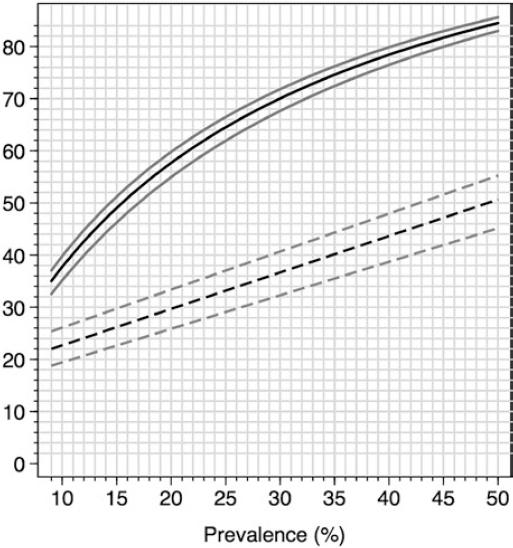
Figure 3 Prevalence of spirometric abnormalities in post-BD spirometry (horizontal axis) and percentage of diagnostic spirometries required in a screening strategy based on a forced expiratory volume in 1 s (FEV1) <87 percentage of predicted (%P) (lower graph, interrupted line with 95% CI) and positive predicted value of a FEV1<87%P (upper graph, continuous line, plus 95% CI). The screening strategy would avoid more than two-thirds of spirometries depending on the prevalence of spirometric abnormalities in the studied population.
DISCUSSION
We studied a population sample with an inexpensive COPD-6 pocket spirometer and with a diagnostic pre- and post-bronchodilator spirometry. We found that pre-bronchodilator FEV1 obtained from the COPD-6 was a good screening test for post-bronchodilator (and pre-bronchodilator) spirometric abnormalities, including restrictive and obstructive, with the best cutoff point at 87%P: a FEV1 ≥87%P would reasonably rule out the requirement of spirometry with few errors, mostly individuals with mild abnormalities.
The availability of adequate spirometry is very limited even in developed countries and having a simple and inexpensive screening test could be of practical value and with high cost-effectiveness, either as a diagnostic tool or as a screening tool. To identify obstruction, a PEF<80%P11 and a crude FEV1/FEV6 <0.8 from 6 s spirometry26 improved efficiency considerably reducing drastically the need of diagnostic spirometries. In the BOLD study, using PEF without a questionnaire was the best option for screening for COPD12. In this study, no combination of symptoms, smoking or other exposures, or previous clinical diagnosis of asthma or COPD improved the performance of FEV1%P from the COPD-6 to identify spirometric abnormalities.
FEV1 is the best-known spirometric prognostic factor and may be reduced by obstructive and restrictive abnormalities, as well as by submaximal inhalation at the beginning of the forced exhalation (poor inspiratory effort). Expressing it as a %P takes into account differences by age, size, and gender. Devices such as the COPD-6 are becoming sophisticated, reliable, and accurate, and correlate well with a diagnostic pre-post bronchodilator test. In addition, a 6 s spirometry shortens the test considerably and reduces the fatigue of patients, especially if a maximum of three maneuvers are performed, with the potential advantage of an even simpler strategy of three maneuvers for at least 1 s each performed with a low-cost instrument.
The restrictive spirometric pattern has been diagnosed inconsistently as a low FVC, defined as an FVC <80%P, or as an FVC <5th percentile (LLN), with a normal FEV1/FVC ratio (FEV1/FVC >0.7 according to the global obstructive lung disease initiative or even better criteria a FEV1/FVC ≥FLLN). Our definition of restriction included a low FEV1 or a low FVC in the absence of airflow obstruction, but for the common definition of a FVC<LLN without obstruction, AUC for FEV1%P was 0.89 (95% CI 0.85-0.96) for spirometries pre- and post-bronchodilator, only slightly lower than that obtained with the definition utilized in this study (AUC = 0.91) (Table 3). The majority (60%) of individuals with a low FVC in the absence of airflow obstruction simultaneously have a low (below LLN) FEV1.
Known determinants of a restrictive spirometric pattern include all true restrictive diseases, that is, those with a reduced total lung capacity (TLC): interstitial lung diseases, abnormalities in the thoracic cage, obesity (important in Mexico), and inspiratory muscle weakness but also conditions with low FVC but normal TLC, such as the non-specific pattern34, air trapping, as often occurs in obstructive diseases, or short expirations, as seen in asthmatic crises, or in individuals lacking the strength or will to perform a prolonged expiration.
Up to 50% of individuals with a physicians diagnosis of COPD lack airflow obstruction (false positives) and may receive unnecessary bronchodilators10,13,35; on the other hand, up to 90% of individuals with airflow obstruction are undiagnosed and may lack appropriate treatment10,13,35. Similar diagnostic problems are likely present for other spirometric abnormalities, and screening with a COPD-6 or similar devices would likely reduce those deficiencies, allowing for a more rational referral to diagnostic spirometry, at a cost of missing a few individuals with mild ventilatory abnormalities, with limited clinical relevance providing all preventive recommendations are made, especially in terms of stopping tobacco smoking.
Limitations of our study included a relatively small sample size and uncommon spirometric abnormalities as Mexico City had the lowest prevalence in the BOLD and PLATINO studies, and we were unable to analyze in detail the performance of the screening instruments by means of the severity of the spirometry abnormality. In a sample of patients from a primary care setting and even more so in patients visiting a referral center, a higher pre-test prevalence of disease is expected and therefore a higher PPV. Of course, the presence of relevant exposures or symptoms, or abnormalities in other tests, considerably increases the pre-test probability of disease and warrants further investigation regardless of the lung function measurements. The strategy should be tested in another population, especially the proposal of requiring only FEV1%P, a maximum or three maneuvers of at least 1 s duration, reducing time, and fatigue. Expected results are good, given the quality of newer devices for measuring FEV1, and even more so with future devices.
In conclusion, our results indicate that screening for spirometric ventilatory abnormalities is improved by a simplified low-cost pocket spirometry test, leaving diagnostic-quality spirometry only for those with a FEV1 of <87% of predicted, that is, one-fourth of the individuals screened in our population-based survey.
SUPPLEMENTARY DATA
Supplementary data are available at Revista de Investigación Clínica online (www.clinicalandtranslationalinvestigation.com). These data are provided by the corresponding author and published online for the benefit of the reader. The contents of supplementary data are the sole responsibility of the authors.
SUPPLEMENTARY MATERIAL










 nueva página del texto (beta)
nueva página del texto (beta)


