INTRODUCTION
Zika virus (ZIKV) belongs to the Flaviviridae family, genus Flavivirus. It is transmitted to humans primarily through the bite of infected mosquitoes such as Aedes aegypti and Aedes albopictus. However, cases of transmission through body fluids, i.e., blood transfusions and sexual contact, have also been recorded1-3. ZIKV infection continues to spread in the Americas following the most recent outbreak in Brazil, starting with the first confirmed case in May 2015, although later evidence showed virus circulation since 20134. Because of this outbreak, several research groups have conducted experimental studies to understand the relationship between ZIKV, and severe fetal abnormalities such as microcephaly and congenital blindness3,5. On the other hand, the disease has been related with the development of Guillain-Barré Syndrome, capable of affecting individuals of any age, and leaving the infected population with long-term sequelae6. Having this in mind, plus the fact that approximately 80% of ZIKV infection cases are asymptomatic7 or oligosymptomatic8, it is crucial to implement timely diagnostic methods associated with a decision-making system, depending on the type of patient and symptomatology. This review is focused on ZIKV diagnostic methods with a particular emphasis on those developed as point-of-care (PoC) systems, considering their application to opportunely identify asymptomatic cases in endemic areas (acute phase of infection) where there is cocirculation of other flaviviruses, and how their use could reduce the time and cost of diagnosis.
ZIKA: STRUCTURE AND GENOME
ZIKV is an enveloped virus with an icosahedral capsid of approximately 50 nm in diameter9,10. Its genome is comprised a positive single-stranded RNA of about 10,794 bp that encodes a single open reading frame (ORF) flanked by two non-coding regions (untranslated region [UTRs]) at 5 and 3 ends. The ORF expresses a polyprotein that post-translationally splits to generate three structural proteins: capsid (C), membrane precursor (prM), and envelope (E); as well as seven non-structural proteins: NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS511,12. The viral RNA is contained in the capsid, surrounded by a lipid bilayer where M and E proteins are anchored through transmembrane domains. These morphological and genomic characteristics are shared with other clinically relevant flaviviruses such as Dengue Virus (DENV), Yellow Fever Virus, Japanese Encephalitis Virus, and West Nile Virus.
Zika E protein is a glycoprotein of 500 aa that forms dimers with an anti-parallel organization on the surface of the virus. E protein plays an essential role in the infection mechanism, as it is involved in receptor-mediated endocytosis. Likewise, the glycosylation pattern is related to the differentiation of the virus lineages. There are two known ZIKV lineages, African and Asian, where the latter is more related to the strains that caused the epidemics in the Americas13. E protein represents an important target for diagnosis, besides two nonstructural proteins, NS1 and NS5.
INFECTION STAGE AND RECOMMENDED DIAGNOSTIC METHODS
Acute phase
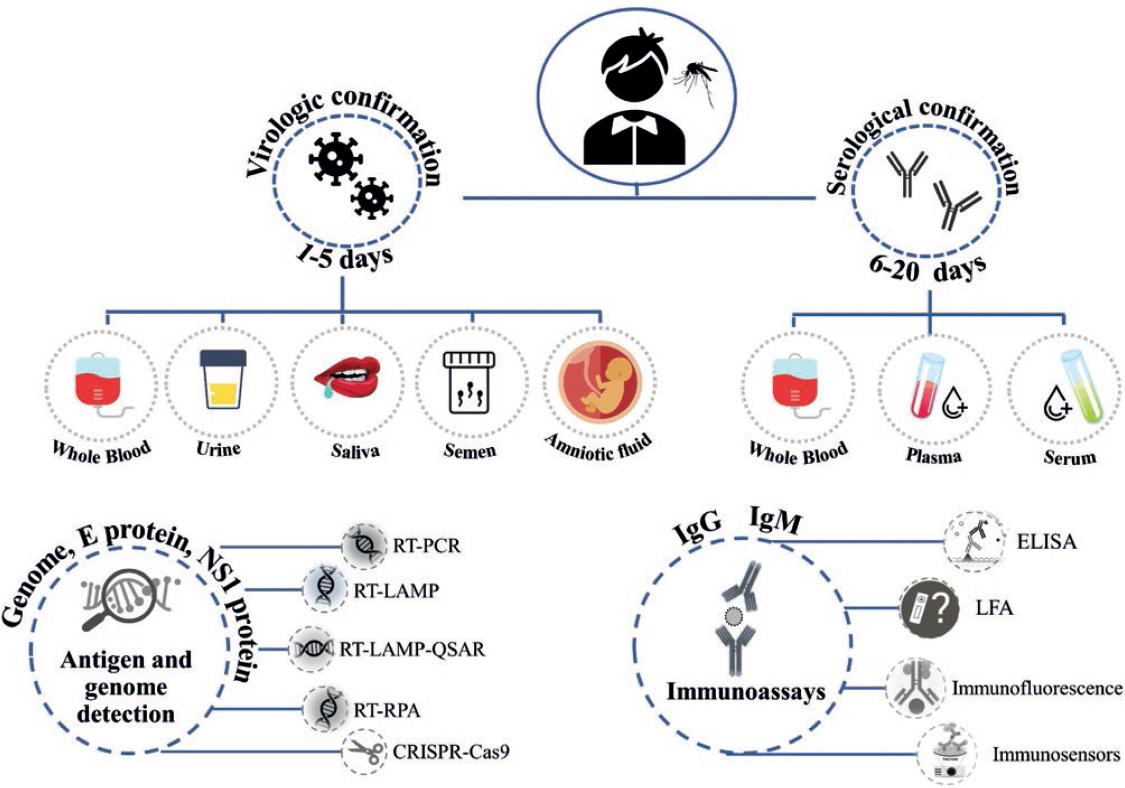
During the first 5 days of infection, known as the acute phase, the virus circulates in the fluids of the patients (viremia). Therefore, direct diagnostic methods that detect viral components such as RNA or proteins are used (Fig. 1). These components are detectable in various body fluids such as whole blood, plasma, serum, urine, saliva, semen, and amniotic fluid14,15 (Fig. 2). Furthermore, during the acute phase, it is possible to perform virus isolation to determine its presence in a sample. However, this process takes more time and requires a biosafety level 2 (BSL2) laboratory; besides, in some countries, this technique is performed only for research and public health surveillance purposes16.
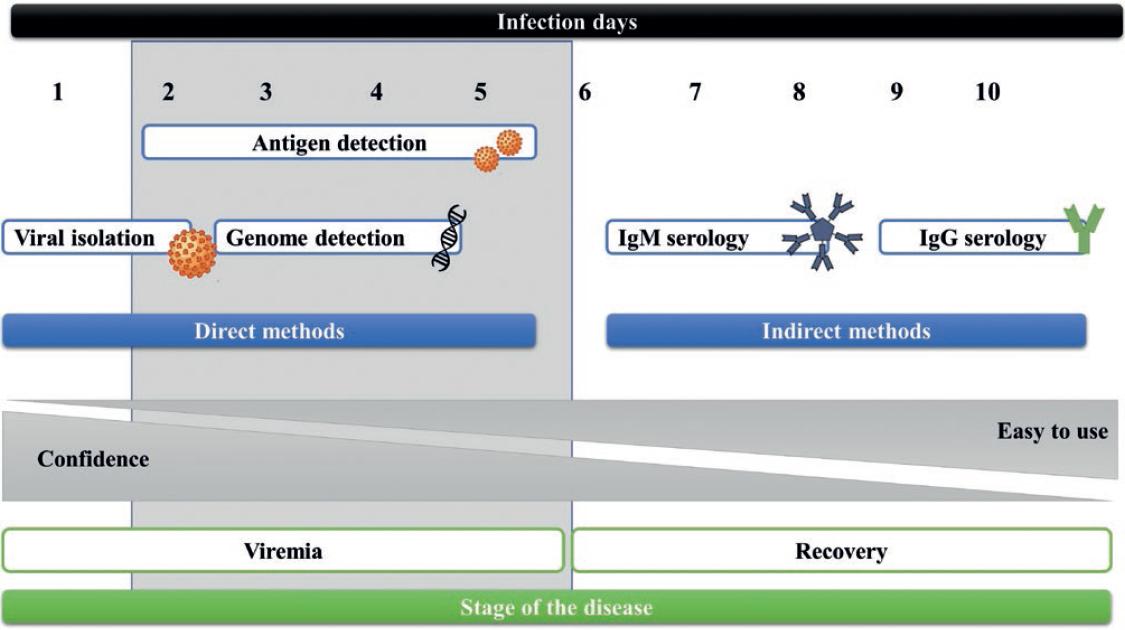
Figure 1 General diagram of Zika virus infection and the most recommended detection methods during the different infection stages.
The standard method for ZIKV diagnosis during the viremic period is the reverse transcription-polymerase chain reaction (RT-PCR), which is highly sensitive and specific; however, the use of this technique has some problems. For example, symptoms are often mild and go unnoticed, and virus concentration decreases rapidly in the different body fluids such as serum (8.1-30 × 106 copies/mL), saliva (0.02-90 × 106 copies/mL), and breast milk (0.0004-2.1 × 106 copies/mL)17,18. Another direct diagnostic method used during the acute phase is enzyme-linked immunosorbent assay (ELISA), which in most cases, is used for the detection of proteins NS1 or E (pE). E protein is present in the virus surface, while NS1 protein plays a role in viral replication, but it is also secreted into the extracellular space as a hexameric form, similar to other flaiviviruses19.
Convalescence phase
ELISA technique and plaque-reduction neutralization test are used for the detection of Immunoglobulin M (IgM) and IgG, respectively. However, in both cases, cross-reaction with other flaviviruses has been reported. The main disadvantage of using these standard methods is that they are time-consuming and require specialized personnel to perform the tests in BSL2 facilities16. During the Zika Strategic Response Plan, the WHO aimed to strengthen the capacity of the different laboratories around the world to test for the virus, since most of the affected regions are mainly developing countries. Keeping this common goal, they generated a first target product profile (TPP), focused on the diagnosis of active ZIKV infection (acute phase), and a second one focused on the diagnosis of prior infection, better described by Chua et al.20
Therefore, this highlights the need for diagnostic devices at the PoC, with some specific characteristics, including limit of detection (LOD) of <50-500 copies/mL, specificity >98%, sensitivity >95-98%, affordability, rapid results, that could be applied in capillary blood or less invasive samples (urine, saliva, or others), and should be ready to use. Table 1 shows diagnostic methods authorized for emergency use by the US Food and Drug Administration (FDA), with the advantage that these tests do not require prior treatment of the sample, which makes them more attractive for the development of PoC platforms.
Table 1 Diagnostics for the viremia stage of ZIKV with emergency approval by the FDA
| Number | Company | Assay format | Biological matrix | Sample volume | Assay time | FDA authorization date | Reference |
|---|---|---|---|---|---|---|---|
| Aptima Zika Virus Assay |
Hologic, Inc. | TMA and HPA | Serum, plasma, processed urine, full blood | 700 μL | 3.5 h | June 17, 2016 | 38 |
| Sentosa SA ZIKV RT-PCR Test |
Vela Diagnostics USA, Inc. | RT-PCR | Serum, plasma EDTA, urine |
250 μL | 3 h | September 23, 2016 | 39 |
| Abbott RealTime Zika |
Abbott Molecular Inc. | RT-PCR | Serum, EDTA plasma, urine, full blood | 350 μL | 6.75 h | November 21, 2016 | 40 |
| Zika ELITe MGB Kit U.S. |
ELITechGroup Inc. | ||||||
| Molecular Diagnostics |
RT-PCR | Serum, plasma EDTA | 200 μL | 2.5 h | December 9, 2016 | 41 | |
| TaqPath Zika Virus Kit |
Thermo Fisher Scientific | RT-PCR | Serum, urine | 300 μL | 3 h | August 2, 2017 | 42 |
| CII-ArboViroPlex rRT-PCR assay | Columbia University | RT-PCR | Serum, urine | 250 μL | 6 h | August 11, 2017 | 43 |
TMA: transcription-mediated amplification, HPA: hybridization protection assay.
Alternative methods based on genome detection
Molecular methods are based on ZIKV genome detection (Fig. 2) and generally target highly conserved regions, such as UTR 5 and 3 regions, or partial sequences of E, C, NS1, NS3m or NS5. As already mentioned, the molecular reference method is the RT-PCR, but other nucleic acid amplification and detection techniques have been developed, such as loop-mediated isothermal amplification (LAMP) and amplification of recombinase polymerase (RPA). Isothermal and enzymatic methods are more straightforward, can generate results in minutes, and sample pretreatment is not required to extract the viral RNA. These advantages enable the development of portable devices for rapid diagnosis (Table 2).
Table 2 Summary of molecular assays, immunoassay, biosensors, and new technologies with point-of-care focus for ZIKV detection, and performance characteristics according to the literature
| Detection technique | Detection limit (LOD) | Biological matrix | Sample volume required | Sample treatment | Target | Rehearsal time | Qualitative or quantitative | Reference |
|---|---|---|---|---|---|---|---|---|
| RT-LAMP/Dot Blot | 2.2 × 103 RNA copies/mL | Saliva | 50 μL | | RNA (capsid) | 11.4 min | Qualitative | 23 |
| RT-RPA | 5 × 102 copies/Rxn | Serum, saliva | 5 μL | RNA isolation | RNA (envelope) | 3.38 min | Quantitative | 24 |
| RT-PCR | 1 copy/μL | Plasma, urine and tap water | | Plasma separation and dilution | | 40 min | Quantitative | 44 |
| CRISPR-Cas9 | 2.8 fM | Plasma | 300 μL | RNA isolation | SNPs | 3 h | Qualitative | 45 |
| Electrochemistry (SIP) | 2 × 10−4 PFU/mL (1 copies/mL) | PBS | 80 μL | Serum separation and dilution (1-10%) | ZIKV | | Semi-quantitative | 27 |
| 5 × 102 | Serum | |||||||
| Immunofluorescence amplified by LSPR | 1.28 fg/mL of NS1 in water 8.2 copies of RNA/mL in water 100 RNA copies/mL in serum | Serum | 20 μL | RNA isolation | NS1 protein | | Quantitative | 31 |
| RT-LAMP -QUASR | 2 PFU/mL | Urine, blood and saliva | Does not Specify | RNA isolation | NS5 protein coding regions | | Quantitative | 46 |
| RT-SIBA | 5000 copies/mL | Lysis buffer | 2 μL | RNA isolation | RNA | < 30 min | Quantitative | 47 |
| Immunoassay (SERS) | 10.92 ng/ mL (LFA) 0.72 ng/ mL (SERS) | Serum | 30 μL | | NS1 protein | - | Qualitative | 32 |
| RT-PCR | 40 copies of RNA/mL 102 copies of RNA/mL | Saliva Urine | 140 μL | RNA isolation | | | Quantitative | 12 |
| RT-LAMP | 1 equivalent genome copy/mL | Urine, serum, mosquitoes | 2 μL of urine or raw or lysate serum 1 mosquito (lysis in 100 μL of PBS) | Mosquito Lysis, RNA isolation | | 30 min | Qualitative | 28 |
| RT-LAMP | 1.42 PFU/mL | Saliva, urine, serum | 10 μL of saliva, urine or serum | | | 30 min | Qualitative | 48 |
| Immunoassay | 0.45 nM | PBS | Doesnt Specify | | NS1 protein | < 30 min | Quantitative | 34 |
| RT-LAMP | 1.56 × 105 PFU/mL | Blood | 8 μL | | NS1 coding region | < 1 h | Semi-quantitative | 49 |
| RT-LAMP-FLA | 1 copy of RNA | Blood | 2 μL | RNA isolation | E -protein coding region | 35 min | Qualitative | 50 |
| Improved electrical detection with nanoparticles | 10 particles/ L | Plasma, urine and semen | 10 mL total blood | Plasma separation and viral lysis | E- protein | | Quantitative | 26 |
| Chemiluminescence electrogenerated | 1 PFU | Urine, plasma | 100 μL | | E-protein | 2 h | Semi-quantitative | 34 |
| Emulsifying agglutination | 100 nM | Has not been tested in samples | - | | NS1 protein | 30 min | Qualitative | 34 |
| Paper-based plasmonic biosensor | 1 ng/mL | Serum | | | IgG & IgM anti-NS1 | | Semi-quantitative | 29 |
(a) RT-LAMP: reverse transcription loop-mediated isothermal amplification, (b) CRISPR: clustered regularly interspaced short palindromic repeats, (c) SIP: surface imprinted polymers, (d) LSPR: localized surface plasmon resonance, (e) SIBA: strand invasion based amplification, (f) LFA: lateral flow assay.
After RT-PCR, one of the most commonly used molecular methods is RT-LAMP. Several research groups have used this technique for ZIKV detection, evaluating the compatibility with different types of samples such as serum, urine, and saliva. Furthermore, they have been evaluating this test so that it is specific to ZIKV and does not present cross-reactivity with other related viruses, such as DENV or Chikungunya virus (CHIKV). One example of this technique is the RT-LAMP assay developed by Kurosaki et al., which can distinguish between the lineages of the virus with the advantage that the detection of RNA in the sample takes an average time of 15 min21. Song and Mauk developed a disposable cassette based on RT-LAMP technology for ZIKV detection. The cassette requires a temperature control device which is chemically heated. This system was evaluated using saliva samples, and results were generated in < 40 min, with a detection limit (LOD) between 50 and 100 plaque-forming units (PFU)/Ml21,22. Sabalza et al. also used RT-LAMP, but coupled to dot-blot. These two assays integrated into a microfluidic cartridge, allow the detection of up to 8.57 × 102 copies of RNA/mL in saliva samples in approximately 15 min23.
Another technique that can be used in PoC is RT-RPA, where the capacity to detect different ZIKV strains has been tested, showing a 100% specificity and 83% sensitivity using clinical samples (serum, whole blood, urine, and semen). This test could detect 5 × 102 copies of RNA in an average of 10 min, compared to RT-PCR, which takes almost 60 min to obtain the same result24.
Methods based on electrochemical changes
A detection strategy based on electrochemical immunosensors has been developed for the early stage of the disease. This type of method can be adapted to PoC devices, as it requires few components. These biosensors are based on the detection of antigens or viral particles, using different techniques. Kaushik et al. developed an immunosensor by immobilizing specific antibodies against the envelope protein of ZIKV on a gold microelectrode. Both the capture and detection of the virus are determined by electrochemical impedance spectroscopy. This method yielded an LOD of 10 pM in an average time of 40 min25.
The electrodes used to identify electrochemical changes can also be printed on various surfaces. The creation of such microchips is an approach to the development of PoC devices. Draz et al. have printed electrodes on a hybrid surface of paper and plastic for the detection of viral particles26. Impedance measurement detects the presence of ZIKV in urine, where the virus is extracted from the sample with magnetic particles coated with antibodies against E protein. This method can detect 101 viral particles/μL in < 1 h.
Another approach that does not involve the use of antibodies is surface printing, a technique employed by Tancharoen et al. for ZIKV detection27. The biosensor consists of a gold electrode coated with a mixture of polymers and graphene oxide compounds. This mixture generates a specific cavity of the virus shape, where viruses from a sample fit in, which causes changes in electrical conductivity. An LOD of up to 2 × 10−4 PFU/mL is reported for this biosensor.
Methods based on immunofluorescence and/or chemiluminescence
Archarya et al.28 reported an immunoassay based on electro-generated chemiluminescence for the ultrasensitive and specific detection of ZIKV in human biological fluids, reporting an LOD of 1 PFU in 100 μL of urine or plasma28.
Methods based on surface plasmons
Jiang et al.29 developed a device based on bioplasmonic paper (BPD), which consists of the use of NS1 protein of ZIKV as a recognition element and gold nanorods as plasmonic transducers29. The main advantages are its low cost and the ability to be adaptable to other biomarkers. In addition, the BPD could be functional after 20°C and 60°C incubation for 1 month using the metal-organic framework technique, facilitating its transport to limited access locations. However, the main disadvantages are that it is based on the detection of IgG and IgM, which are known to cross-react with other flaviviruses, and that these biomarkers are outside the timeframe of the acute phase of ZIKV infection29.
Adegoke et al.30 demonstrated that localized surface plasmon resonance (LSPR) signals from plasmonic nanoparticles (NP) can be used to mediate the fluorescence signal of quantum dot nanocrystals in a molecular beacon biosensor probe for ZIKV RNA detection, obtaining an LOD of 1.7 copies/mL, where ZIKV RNA LOD is proportional to the LSPR-mediated fluorescence signal30. Another approach based on LSPR, developed by Takemura et al., comprises an immunofluorescence biosensor for the detection of ZIKV NS1 protein, by means of LSPR of gold NP waves, (AuNPs) demonstrating an LOD up to 8.2 copies/mL31.
Sánchez-Purrá et al.32 integrated a surface-enhanced Raman scattering (SERS)-based lateral flow assay (LFA) immunoassay for simultaneous and differential detection of ZIKV and DENV. The immunoassay consists of a sandwich of polyclonal antibodies immobilized on the test lines, with the ability to recognize ZIKV and DENV NS1 protein; then, a conjugated set of antibodies with Nano-Gold Stars (GNS) is used to develop the reaction. The colorimetric assay interpretation is performed with the naked eye, with an LOD of 10.92 ng/mL, which is in the range of a typical LFA. Nevertheless, the main contribution of this work was the combination of LFA-SERS, using GNS to perform test line measurements in order to obtain SERS spectra, allowing detection of 0.72 ng/mL of ZIKV NS1 and 7.67 ng/mL of DENV NS1 protein32.
Other strategies
Afsahi et al.33 developed a portable biosensor also for the early stage of virus detection. This biosensor uses monoclonal antibodies targeting NS1 protein, which are covalently attached to a graphene surface. All these components together allow quantitative detection in real-time in < 30 min. For this approach, an LOD of 0.45 nM was reported33.
On the other hand, agglutination-based strategies have been developed, demonstrating outstanding results. Zhang et al.34 reported a Janus emulsion agglutination assay for the detection of interfacial protein-protein interactions reduced-charged Sso7d and ZIKV NS1 protein. The rcSso7 replaces the monoclonal antibody use, but keeps the bonding surface. The agglutination assay yields a LOD of 100 nM for ZIKV NS1 protein34.
Hsu et al.35 propose a PoC immunosensor test based on artificial nanozyme platinum/gold core-shell NP (Pt/AuNPs); this device can specifically detect ZIKV in whole-blood without cross-reaction with other flaviviruses such as DENV. Furthermore, this PoC could be used by the patient, following a simple procedure using a drop of whole blood, and the result is quantified with a smartphone algorithm based on grayscale values, avoiding instability of colorimetric signals developed by enzyme reactions35.
CHALLENGES AND PERSPECTIVE
Several and crucial challenges need to be solved to guarantee specific, sensitive, and cost-effective ZIKV detection and diagnosis. It is necessary to consider the suggestions of the TPP2 list issued by the WHO, to simplify sample analysis using whole blood. The individual patient care might benefit from tests performed in a routine diagnostic laboratory, avoiding the use of more sophisticated tests, to confirm cases, such as neutralization assays36.
In addition, a functional multiplex assay that allows simultaneous detection of several flaviviruses (ZIKV, DENV, and CHIKV) could be advantageous because of viral cocirculation in low and middle-income countries, although validation in a large cohort is essential to avoid unspecific results. However, because virus cocirculation varies in different regions of the world, the fact that multiple diagnostic devices could be integrated into a single platform that easily adapts according to the needs of each locality, could be optimal for disease control and epidemiological surveillance. Furthermore, the projected manufacturing costs, storage, transportation, and use requirements of such a platform should adapt to the public health facility users.
In addition, the development of multiple diagnostic tests based on the detection of viremia and circulating antibodies against the virus could provide a comprehensive view of the patients health, spanning the entire course of ZIKV infection. This would be especially useful for diagnostic algorithms implemented by the health authorities.
At present, some commercial or in development PoC tests are focused on the detection of viral RNA copies (rc/mL) or PFU/mL. However, all of them have limitations since these approaches do not represent virus infectivity and require further processing for the determination of infective virions, implying the use of laboratory facilities with BSL 2, thus increasing time and cost37.
Now, the optimization for the detection signal of the virus in serum, saliva, urine, or blood samples, using technologies such as biosensors, agglutination, microfluidics, and paper-based microfluidics, among others, appear to be the best strategy. The LOD obtained using these techniques has proven that they can detect even one viral particle per milliliter. For these new technologies, the next step would be their manufacturing and validation in a significant cohort and on different field-test environments. For this reason, the challenge is to guarantee the researcher the access to clinical sample banks previously characterized, because it could represent a significant advantage in the validation process, decreasing the time spent in sample collection. This could be an improvement for all tests in the development phase, consolidating standardized methods, and avoiding detection mistakes.
The implementation of PoC screening technologies in low and middle-income countries and their cost-effectiveness in epidemic hot spots, such as airports and endemic areas, could control the dissemination of ZIKV and allow rapid management of infected patients. The implementation of these strategies would represent the first step to change the classic paradigm for testing ZIKV algorithms (and many others), where PoC tests could be considered as a valid screening approach to cut viral spreading during epidemic outbreaks in the near term.
CONCLUSIONS
Here, we examined several alternatives for ZIKV diagnosis reported in the literature. Undoubtedly, substantial progress has been achieved in detecting the virus. The current challenge is to integrate these advances to the development of portable devices for implementation at the PoC in the acute phase. Another considerable challenge is the development of a differential diagnosis between cocirculating viruses, mainly in endemic regions where DENV and CHIKV are present simultaneously. These creative approaches will definitively improve the cost-effectiveness ratio of laboratory tests currently implemented by the health sector in Latin American countries.











 nova página do texto(beta)
nova página do texto(beta)