Letter to the editor
Operational Recommendations for the Attention and Prevention of
SARS-CoV-2 Infection at Interventional Bronchoscopy Units
Olivia Sánchez-Cabral1
Dina Martínez-Mendoza2
Miguel Á Ramírez-Candelas1
Krizia J. Jassen-Avellaneda1
Silvia D. Ponce-Campos1
Maria de los Ángeles Macías-Jiménez1
Cira Santillán-Díaz1
*
1Interventional Pulmonology Unit and National
Institute of Respiratory Diseases (INER) “Ismael Cosío Villegas”, Mexico City,
Mexico
2Epidemiology Surveillance Unit, National
Institute of Respiratory Diseases (INER) “Ismael Cosío Villegas”, Mexico City,
Mexico
Dear Editor
Highly specialized bronchoscopic procedures performed at Interventional Pulmonology Units
are considered high risk due to the particle aerosolization. In response to the current
world health situation, due to the infection of the new severe acute respiratory
syndrome coronavirus and coronavirus disease (COVID-19) and despite the fact that
bronchoscopy has a relatively contraindicated indication due to the risk of infection in
health personnel and for its limited usefulness in COVID-19 diagnosis, is important to
establish protocols on how to act in the operating centers for patients care with
COVID-19, as well as for the prevention of infection in health personnel. The document
shown in supplementary information contains recommendations proposed by experts from the
Interventional Pulmonology Unit of the National Institute of Respiratory Diseases
“Ismael Cosío Villegas” in Mexico City and by International Organizations. As shown in
the guideline, the most important points to consider are as follows: prioritization of
procedures, patient care, staff distribution, description of work areas, procedure room
conditions, patient transfer, intervention flowchart, personal protection, and
processing of bronchoscopy equipment.
SUPPLEMENTARY DATA
Supplementary data are available at Revista de Investigación Clínica online
(www.clinicalandtranslationalinvestigation.com). These data are
provided by the corresponding author and published online for the benefit of the
reader. The contents of supplementary data are the sole responsibility of the
authors.
PRIORITIZATION OF PROCEDURES
During the severe acute respiratory syndrome coronavirus 2 pandemic, elective
procedures will be suspended, only urgent procedures will be performed.
ATTENTION OF PATIENTS WITH CORONAVIRUS DISEASE 2019 (COVID-19)
During the pandemic, all patients should be considered COVID-19
positive
Nasopharyngeal, oropharyngeal swabs, and tracheal aspirates (already
performed by the treating service) should be the diagnostic method of
choice
-
Bronchoscopy is a relatively contraindicated procedure; it has limited
utility in the diagnosis of COVID-19 since it is a high-risk procedure
for personnel and should only be considered in the following
scenarios1-7:
To have negative nasopharyngeal, oropharyngeal, and tracheal
aspirate tests
If there is an alternative diagnosis that modifies the
treatment
Life-threatening conditions: airway obstruction or massive
hemoptysis
Aspiration of secretions that compromise ventilatory
mechanics
Percutaneous tracheostomy.
All hospitalized patients with an indication for orotracheal intubation
during the shift, according to the clinical context, will be transferred
to the bronchoscopy unit, where they will proceed to perform orotracheal
intubation using a rapid intubation sequence, as well as a
bronchioloalveolar lavage (in case of having indication). If you cannot
transfer the patient, you must have a portable computer.
The rapid intubation sequence to be performed is proposed below8-12:
The patient wears surgical mask until induction begins
-
Preoxygenation for 5 min with 100% of fractional of inspiration oxygen
and monitoring of vital signs
Rapid intubation sequence will be performed (fentanyl, midazolam,
rocuronium, or succinylcholine) no ventilation
Ensure deep neuromuscular block using neuromuscular transmission
monitoring
Trendelenburg position is given to the patient, and the bronchoscopist
will be notified
The bronchoscopist will perform intubation with indirect visualization
using rigid lens intubation technique (this technique minimizes the
risks of infection of health personnel related to intubation), verifying
the appropriate placement
The balloon of the tube is inflated
Connect to the anesthetic circuit with a high-efficiency filter to start
mechanical ventilation, which will be carried out according to the
established protocol
The patient will be ventilated to stabilize SaO2 (arterial
oxygen saturation) and ETCO2 (exhaled carbon dioxide), for at
least 3 min
Mechanical ventilation is suspended
Bronchoalveolar lavage will be performed in apnea (if indicated)
At the end of the wash, the endotracheal tube is reconnected to the
anesthetic circuit.
The sample must be compulsorily taken by trained personnel and must be considered
highly infectious, so it is essential to wear personal protective equipment7. The sample types are shown in Table 1.
Table 1 Description of the sample types7
| Type of sample |
Material |
Transport temperature |
Storage |
Comments |
| Pharyngeal and nasopharyngeal exudate |
Viral transport medium Dacron or rayon swabs with
plastic handles (pharyngeal exudate) Dacron or rayon swabs with
flexible handle (nasopharyngeal exudate) |
2 - 8°C |
≤ 5 days: 2 - 8°C > 5 days: −70°C |
Pharyngeal and nasopharyngeal exudate should be placed
in the same tube to increase viral load. |
| Washed bronchioalveolar |
Sterile container with viral transport medium |
2 - 8°C |
≤ 48 h: 2 - 8°C > 48 h: −70°C |
There may be dilution of the pathogen, but it is still
worth taking. A minimum of 2 ml is required (1 ml of
bronchioalveolar lavage plus 1 ml of transport medium). |
| Tracheal aspirate, nasopharyngeal aspirate, or nasal
wash |
Sterile container with viral transport medium |
2 - 8°C |
≤ 48 h: 2 - 8°C > 48 h: −70°C |
A minimum of 2 ml is required (1 ml of aspirate, plus 1
ml of transport medium). |
| Lung biopsy |
Sterile container with viral transport medium |
2 - 8°C |
≤ 5 days: 2 - 8°C > 5 days: −70°C |
2 cm3 from the visibly most affected
part. |
Table 2 Description of the work areas
| Description of work areas |
|---|
|
|---|
| Area |
Description activities |
| Dressing rooms |
Site intended for the removal of cloth-ing and personal
accessories as well as the placement of disposable surgi-cal
suits. |
| Uncontaminated area |
• Computer area for administrative processes and case
discussion. |
|
• Location and placement area of personal protective
equipment. |
| Contaminated area |
• Patient transfer hall. |
|
• Equipment washing area. |
|
• Procedure room. |
Flowchart of work in the interventional bronchoscopy unit
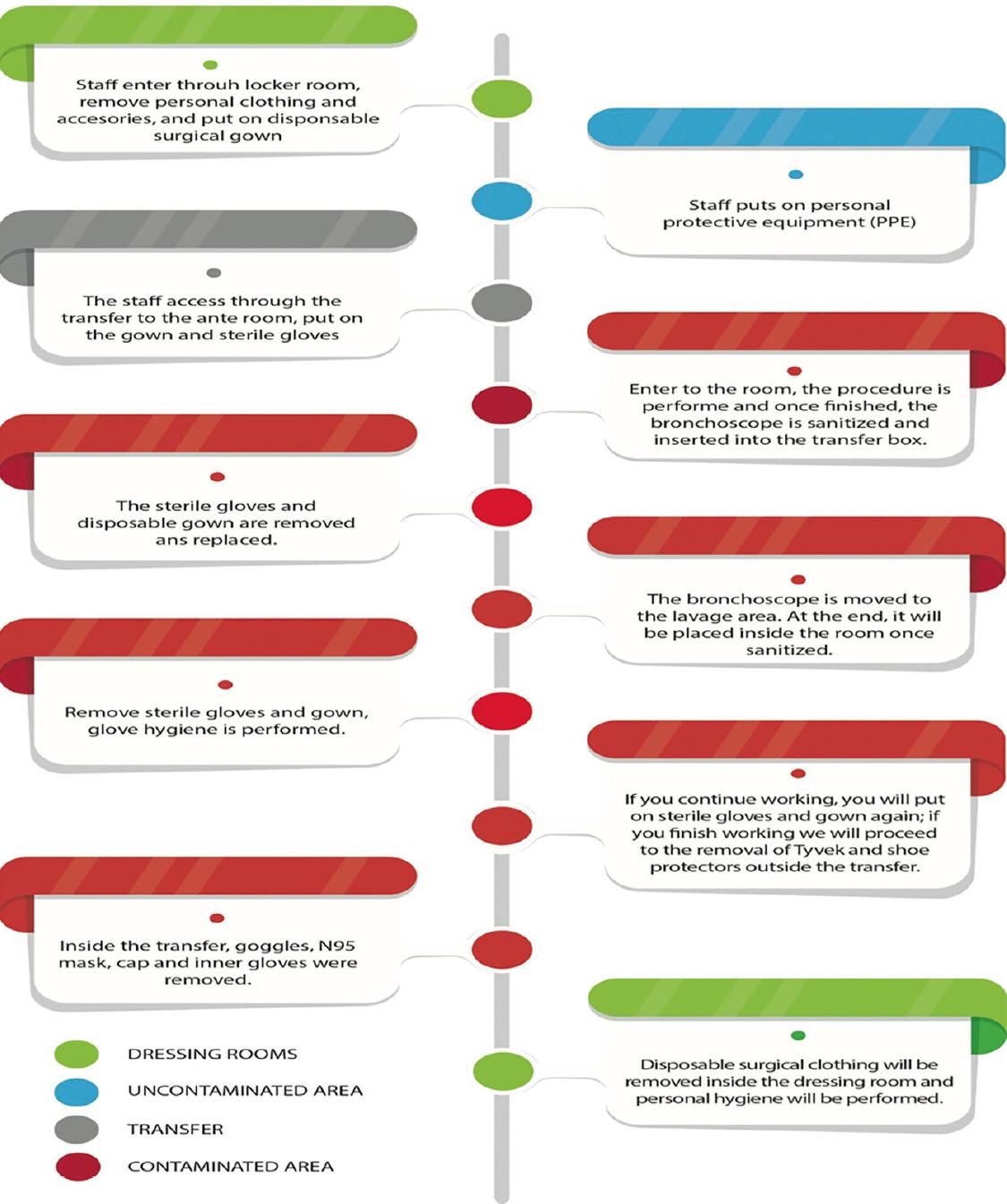
DISTRIBUTION OF EQUIPMENT WITHIN THE UNIT
Equipment distribution:
-
Personnel inside the operating room (performing the procedure):
Interventional/bronchoscopist and anesthesiologist.
-
Equipment washing (bronchoscope and equipment in general)
Washing support personnel.
Medical work office
-
Warehouse
GENERAL RECOMMENDATIONS OF THE PROCEDURE ROOM
The bronchoscopy room must have negative pressure, as well as minimum air changes
of> 12 times/h. The air must be removed directly to the outside or be strictly
monitored by the highly efficient filtration system for particles in the air, before
recirculation. It must be monitored and documented by the institution’s infection
control personnel13.
GENERAL RECOMMENDATIONS FOR STAFF1,3,4,13,14
All personnel must wear a surgical mask at all times.
Maintain a distance of at least 2 m between staff and patients (if
possible)
Personnel must wash their hands with soap and water or disinfect with
alcohol gel before and after contact with the patient, as well as before
and after each procedure
Limit personnel that are in contact with the patient (preferably two)
Minimize contact time with the patient
All personnel must wear the following equipment: cap, gloves, surgical
masks with face mask, N95 mask, surgical suit and heat-sealed
(disposable) protective suit, and shoe protector
The room must remain 30 min alone after the procedure, and it will be
exhaustive and disinfected
The equipment will be limited to what is strictly necessary inside the
room
At the end of the procedure, the patient will leave the operating room
directly to his treating service.
GENERAL RECOMMENDATIONS FOR THE TRANSFER OF THE PATIENT
The patient must be transferred by the team and medical personnel in
charge
Patients will enter and exit through the main door of the unit, directly
to the room
At the end of the procedure, you will be discharged directly to your
clinical service, accompanied by medical personnel and transfer
equipment
No more than 1 patient will be allowed in the unit simultaneously, so
there will be no recovery room.
BRONCHOSCOPE REPROCESSING
-
Mechanical washing
Start immediately after the procedure to avoid drying or
hardening of organic residues
They must wear full personal protective equipment
The outside part of the bronchoscope should be cleaned with a
gauze soaked with 75% alcohol and suck it through the
channel
Suction ports and accessories must be separated, before leak
test
The bronchoscope will be placed in an airtight polyethylene
bag, to be transferred from the procedure room to the
washing area
Perform a leak test (pressurized instrument, with water). Its
presence indicates a violation of the integrity of its
external or luminal part. Must be repaired before reuse
Immerse the bronchoscope in enzymatic soap (according to the
characteristics of each bronchoscope) for approximately 5
min
The external surface must be cleaned manually with the
enzymatic detergent, then use a cleaning brush through all
the ports (perform several times until no organic debris is
observed and discard)
Rinse all channels with the same enzyme soap
Rinse external part and channels with water to remove the
enzymatic cleaner and prepare for disinfection.
-
Disinfection.
According to the above, the bronchoscope is a semi-critical
device (devices that come into contact with intact mucous
membranes and do not normally penetrate the sterile tissue),
therefore requiring high-level disinfection
Disinfection with orthophthaldehyde will be performed
Disinfection for 20 min in 2% alkaline glutaraldehyde at 20°C
provides adequate disinfection, if before this use
detergent
In general, solutions can be reused for 14-28 days
The potency of the solution must be periodically tested by
commercially available test kits (must be discarded if the
concentration is less than 2%)
The solution must be tested at the beginning of each day of
use
After disinfection or sterilization, rinse the bronchoscope
and internal canal with sterile water
Ideally, the instrument dries by purging the canal with 70%
alcohol and compressed air
Flexible bronchoscopes should be hung valveless vertically in
a spacious cabinet with adequate ventilation to prevent
moisture
Do not store them in cases that cannot be disinfected13.
In conclusion, due to the high risk of infection that bronchoscopic procedures
perform during the COVID-19 pandemic in health personnel, it is essential that the
interventional bronchoscopy units have action protocols. The most important points
to consider are as follows: prioritization of procedures, patient care, staff
distribution, description of work areas, procedure room conditions, patient
transfer, intervention flowchart, personal protection, and processing of
bronchoscopy equipment.
REFERENCES
1. Wahidi MM, Lamb C, Murgu S, Musani A, Shojaee S, Sachdeva A, et
al. American association for bronchology and interventional pulmonology (AABIP)
statement on the use of bronchoscopy and respiratory specimen collection in
patients with suspected or confirmed COVID-19 infection. J Bronchol Interv
Pulmonol. 2020;4:1-4.
[ Links ]
2. Infection Control. Disinfection of Healthcare Equipment.
Guideline for Disinfection and Sterilization in Healthcare Facilities;2008.
Available
from:https://www.cdc.gov/infectioncontrol/guidelines/disinfection/healthcare-equipment.html.
[Last accessed on 2020 Apr 03].
[ Links ]
3. Zhejiang University School of Medicine, Alibaba and the Jack Ma
Foundation. Handbook of COVID-19 Prevention and Treatment Compiled According to
Clinical Experience. China:Zhejiang University School of Medicine, Alibaba and
the Jack Ma Foundation;2020.
[ Links ]
4. Joseph T, Ashkan MM. International Pulmonologist’s Consensus on
COVID-19. India:Amrita Institute of Medical
Sciences;2020.</p>
[ Links ]
5. Coronavirus Disease 2019. Clinical Care Guidance. Interim
Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease
(COVID-19). Available
from:https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html.
[Last accessed on 2020 Apr 03].
[ Links ]
6. Surgical and Procedural Guidelines. UMMC ICU Endoscopy Protocol
During COVID-19 Pandemic. Available
from:https://www.umc.edu/CoronaVirus/Mississippi-Health-Care-Professionals/Clinical-Resources/SurgicalandProceduralGuidelines/ICU-GI-Pulmonary-Endoscopy-Process.html.
[Last accessed on 2020 Apr 03].
[ Links ]
7. Dirección General de Epidemiología. Lineamiento Estandarizado
Para la Vigilancia Epidemiológica y Por Laboratorios de COVID-19.
Mexico:Dirección General de Epidemiología;2016.</p>
[ Links ]
8. Greenland JR, Michelow MD, Wang L, London MJ. COVID-19
Infection:implications for perioperative and critical care physicians.
Anesthesiology. 2020;132:1346-61.
[ Links ]
9. Meng L, Qiu H, Wan L, Ai Y, Xue Z, Guo Q, et al. Intubation and
ventilation amid the COVID-19 outbreak:wuhan’s experience. Anesthesiology.
2020;132:1317-32.
[ Links ]
10. Luo M, Cao S, Wei L, Tang R, Hong S, et al. Precautions for
intubating patients with COVID-19. Anesthesiology.
2020;132:1616-8.</p>
[ Links ]
11. World Health Organization. Confederación Latinoamericana de
Sociedades de Anestesiología, Protocolo COVID-19. Geneva:World Health
Organization;2020.
[ Links ]
12. Peng PW, Ho PL, Hota SS. Outbreak of a new coronavirus:what
anaesthetists should know. Br J Anaesth. 2020;124:497-501.
[ Links ]
13. Mehta AC, Prakash UB, Garland R, Haponik E, Moses L, Schaffner
W, et al. American college of chest physicians and American association for
bronchoscopy concensus statement:prevention of flexible bronchoscopy-associated
infection. Chest. 2005;128:1742-55.
[ Links ]
14. Báez MM. Protocolo de Bioseguridad y Biocustodia Para el Manejo
de Pacientes Durante la Toma de Muestras de Casos Sospechosos de Enfermerdad por
2019-nCov. Mexico:Instituto de Diagnóstico y Referencia
Epidemiológicos;2020.
[ Links ]
Copyright: © 2020 Revista de Investigación
Clínica.











 nueva página del texto (beta)
nueva página del texto (beta)



