INTRODUCTION
The outbreak of the novel coronavirus disease (COVID-19) caused by the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is indeed a pandemic threat to global public health. Although the pandemic’s epicenter was initially located in Wuhan, China, in December 2019, the disease has spread worldwide and millions of lives have been affected not only by the disease but also by the economic consequences of compulsory isolation/quarantine1.
To date, no specific therapeutic agents or vaccines for COVID-19 are available; several options such as remdesivir, hydroxychloroquine, lopinavir/ritonavir, and others are currently under investigation, but their antiviral efficacy has not been fully proven2. The use of convalescent plasma (CoPla) was recommended as empirical treatment of Ebola in 2014 and later, in the management of the Middle-East respiratory syndrome, SARS-CoV, H5N1 avian influenza, and H1N1 influenza3,4. CoPla has been recently used in the treatment of patients with COVID-19 in China3-5 and was found to be safe and effective; in addition, it is affordable, a key requirement in low- and middle-income economies6.
Based on the above-mentioned concepts, we designed a protocol to treat patients with severe forms of COVID-19 in México with CoPla and registered it in www.clinicaltrials.gov NCT4357106 after approval by the Ethics Committee of the Clínica Ruiz (number CEI-03-04-20-01). The results obtained in this pilot study of 10 patients are presented.
MATERIALS AND METHODS
This study was conducted in the Centro de Hematología y Medicina Interna, of the Clínica Ruiz in Puebla, Mexico, between April 17, 2020, and May 8, 2020. After its approval by the ethics committee, patients and donors provided written informed consent to participate in the study. The study is prospective, longitudinal, single arm, and quasi experimental.
Patients
Patients with confirmed laboratory evidence of COVID-19 by reverse transcriptase-polymerase chain reaction (RT-PCR) of the upper respiratory tract secretions obtained by swab test, were eligible to receive CoPla provided that they fulfilled the following criteria: (a) severe pneumonia with rapid progression; (b) PaO2/FiO2 < 300, with or without mechanical ventilation support; (c) admitted to the intensive care unit (ICU); (d) age above 18 years; and (e) willing to participate and having signed the informed consent form – either patients or first-degree relatives. ABO blood types were determined for donor compatibility and each patient received 200 ml of ABO-compatible CoPla. The patients were also administered the necessary treatment established by the ICU physicians.
Donors
Donors with laboratory confirmed COVID-19 by RT-PCR of the upper respiratory tract secretions obtained by swab test were eligible, provided that they fulfilled the following criteria: (a) positive RT-PCR test at diagnosis and (b) negative RT-PCR test 10 days after the disappearance of symptoms of the disease, performed twice with a difference of 24 h. The mandatory pre-donation tests in Mexico were also required to be negative and included: hepatitis B virus, hepatitis C virus, HIV, Brucella sp., and syphilis. Apheresis procedures were conducted in all donors with an Amicus machine (Fresenius Kabi, Deerfield, IL, USA) or a Spectra Optia machine (Terumo BCT, Lakewood, CO, USA) and following the Spin-Nebraska protocol7. Two-hundred milliliter aliquots were prepared and frozen.
Clinical information
The following information was collected from each patient: days to admission from symptom onset, presenting symptoms, concomitant treatments, mechanical ventilation, body temperature, PaO2/FiO2, sequential organ failure assessment (SOFA) score, complete blood cell count, liver and kidney function tests, C-reactive protein (CRP), D-dimer, and chest imaging studies.
RT-PCR
Nasopharyngeal/oropharyngeal specimens were collected in a viral transport medium during hospitalization and forwarded to the laboratory. The qRT-PCR for SARS-CoV-2 was performed according to the published protocols of the US-Centers for Disease Control and Prevention (CDC), Atlanta (CDC 2019-nCoV Real-Time RT-PCR Diagnostic Panel)8 and/or Charité Virology, Berlin (Diagnostic detection of 2019-nCoV by real-time RT-PCR)9; the latter was conducted with a RdRP probe modified by the national reference laboratory, Instituto de Diagnóstico y Referencia Epidemiológicos, Mexico. Briefly, total nucleic acid extraction from the samples was conducted with the QIAamp RNA Viral Kit (Qiagen, Mexico), and the qRT-PCR was performed with the SuperScript™ III One-Step RT-PCR System with Platinum™ Taq DNA Polymerase kit (Thermo Fisher, Mexico), and primers and probes specific for SARS-CoV-2 as outlined above8,9.
Anti-SARS-CoV-2 antibodies
Immunoglobulin G (IgG) and IgM anti-coronavirus antibodies were determined by rapid lateral flow immunoassay after apheresis, according to the manufacturer’s instructions: 5-20 µl of total blood were placed in the sample well, 80-100 µl of the manufacturer’s buffer were placed in an adjacent well, and both were incubated at room temperature for 10 min: a positive result was reflected by the appearance of a color band in the control and in the IgG and/or IgM indicators10. Each cassette included a positive control and the results were confirmed by qualitative enzyme-linked immunosorbent assay assessment; the neutralizing activity of the antibodies was not assessed.
RESULTS
Patients
Ten male patients with a median age of 53 years (range 27-72) were prospectively included in the study. All were in the ICU of three hospitals in Puebla, Mexico. Table 1 summarizes the patients’ salient features. They all harbored SARS-CoV-2 virus in the upper respiratory tract identified by RT-PCR. When the CoPla was infused, the median PaO2/FiO2 ratio (Kirby index) was 124 (range 59-221). Supplemental oxygen was being actively administered to all patients, but 5 cases were on mechanical ventilation support; 4 were febrile and the median SOFA score was 3 (range 1-11). The levels of CRP, D-dimer, and ferritin levels were obtained and are shown in figure 1, which also shows the evolution of the patients’ SOFA score, Kirby index, and body temperature after the infusion of CoPla. Over a period of 8 days, the patients’ SOFA score dropped significantly from 3 to 1.5 (p = 0.014); the Kirby index increased from 124 to 255 (p < 0.0001), and body temperature dropped from 38.1°C to 36.9°C (p = 0.0058). Chest X-rays films improved in 7 of 10 patients and in 6, computerized tomography (CT) scans also revealed improvement of the lung injury (Fig. 3). These clinical findings clearly reflect an improvement in overall respiratory function and clinical condition of the patients after the CoPla infusion; accordingly, three out of five cases on mechanical ventilation were able to be extubated, nine were transferred from the ICU to the hospital’s conventional floors, and six were sent to their homes. One patient (number 2) developed a fatal pulmonary embolism on the day of hospital discharge after having recovered from COVID-19; he was not receiving anticoagulants. Patient number 9 died after being transferred to the ICU of another hospital with more limited resources. Figure 2 shows the patients’ overall survival using the Kaplan–Meier method11. The administration of allogeneic plasma had no side effects, including allergic or hemolytic reactions.
Table 1 Salient features of the patients
| Patient | Age | Mechanical ventilation | Days | Comorbidities | Treatment prior CoPla |
|---|---|---|---|---|---|
| 1 | 72 | Yes | 8 | DM, HT | ST, HY, AZI |
| 2 | 54 | No | 6 | DM, HT | ST, HY |
| 3 | 53 | No | 7 | DM, OB | ST, HY, AZI |
| 4 | 64 | No | 4 | OB | ST, HY, AZI |
| 5 | 43 | Yes | 10 | ST, HY | |
| 6 | 46 | Yes | 4 | ST, HY, AZI, TOC, LOP/RIT | |
| 7 | 27 | Yes | 2 | DM, OB | ST, HY, TOC, LOP/RIT |
| 8 | 64 | No | 1 | HT | ST, HY |
| 9 | 59 | Yes | 1 | DM, OB | ST, HY, AZI |
| 10 | 38 | No | 2 | DM, OB | ST, HY, AZI, LOP/RIT |
Days: number of days in the ICU before the administration of plasma; DM: diabetes mellitus; HT: hypertension; OB: obesity; ST: steroids;
HY: hydroxychloroquine; AZI: azithromycin; TOC: tocilizumab; LOP/RIT: lopinavir/ritonavir; CoPla: convalescent plasma.
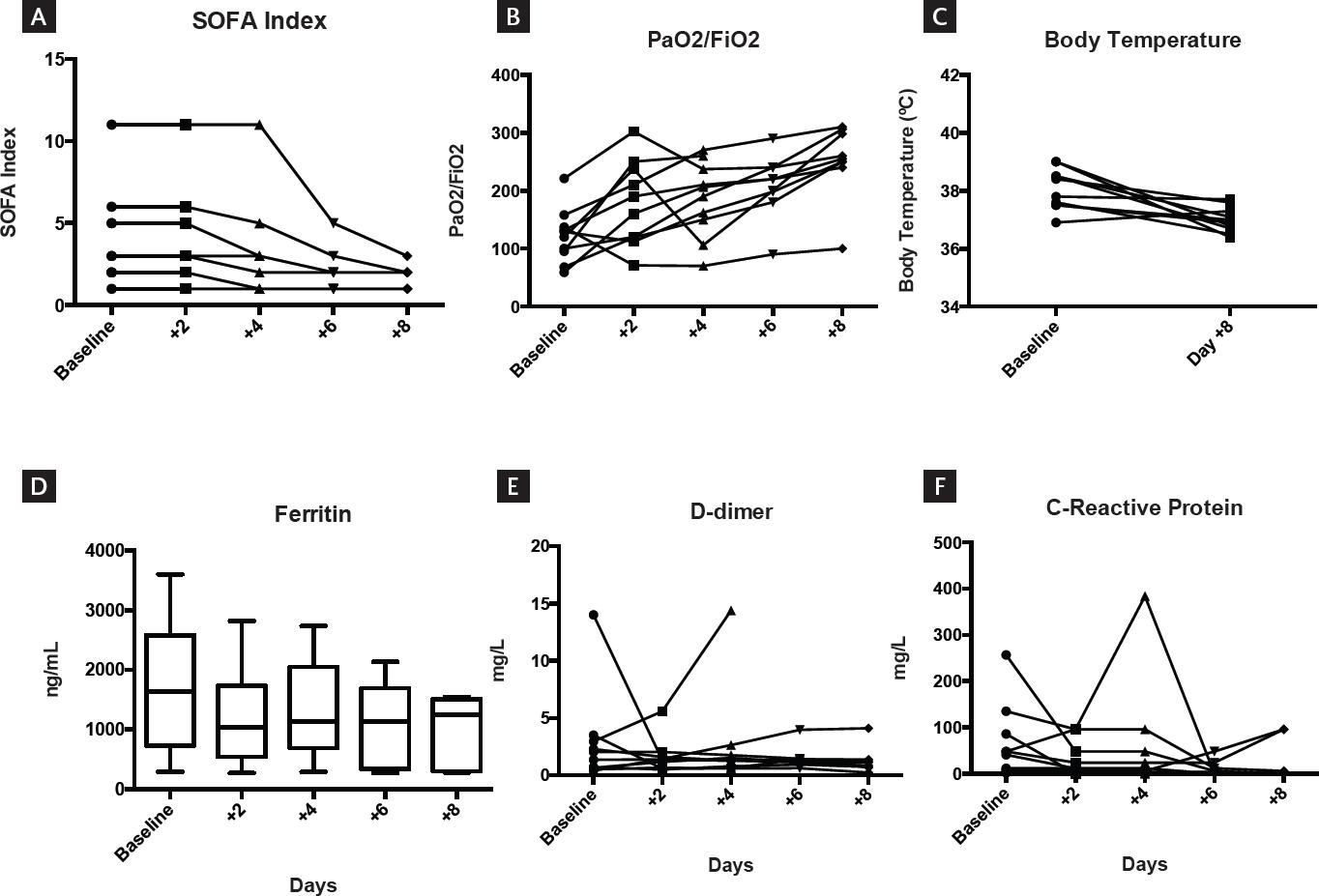
Figure 1 Changes after plasma infusion: panel A depicts the significant drop of the sequential organ failure assessment index; panel B shows the significant increase in the Kirby index (pO2/FiO2); panel C shows the significant decrease in body temperature; and panel D shows the significant drop in serum ferritin levels. Panels E and F indicate the drops in D-dimers and C-reactive protein, which are not significant.
Donors
CoPla’s was obtained from five donors with a median age of 35 years (range 24-52), two of which were female. All were negative to SARS-CoV-2 in the upper respiratory tract in at least two consecutive tests obtained 24 h apart and before donation. Four donors had IgG anti-SARS-CoV-2 IgG while one had anti-SARS-CoV-2 IgM. The median volume of plasma obtained from the donors was 700 ml, range 600-1000 ml. In all instances, the plasma matched the patient’s ABO group. Bacterial cultures of all plasma samples were negative.
DISCUSSION
The previous studies have reported the merit of CoPla in several infectious diseases2-5 and specifically, in COVID-193,12. In this study, 10 critically ill patients due to infection by the SARS-CoV-2 virus and with a Kirby index below 300 reflecting severe pulmonary injury received CoPla from five donors. All patients had a positive result, defined as the improvement in both their clinical course and in the surrogate markers of the disease; three-fifth of patients on mechanical ventilation no longer required respiratory support 10 days after transfusion of the CoPla. The patient overall survival was 77%, 24 days after infusion of the CoPla, and the two deaths could have strictly been avoided: one, if he had received anticoagulation therapy and the other, had he not been transferred to another ICU. The changes in the surrogate markers of disease activity suggest a beneficial effect of the plasma infusion: there were significant improvements in body temperature, in the Kirby index (PaO2/FiO2), the SOFA index, and in the serum ferritin levels. We did not determine the patient viral loads.
Since the patients were receiving other treatments (Table 1), it is difficult to conclude that the CoPla infusion was fully responsible for the improvement in pulmonary function and in the patients’ clinical course. It is clear that our pilot study has several limitations: (1) the number of patients is low; (2) the results cannot be generalized; (3) there is no control group; (4) there are several confounding factors, mainly the other treatments administered in conjunction with the CoPla; and (5) the inclusion criteria are limited since only severely ill patients were included.
Be that as it may, this pilot study suggests that the administration of CoPla may result in improvement in both respiratory function and in the clinical course of COVID-19 patients. Although the numbers are small, the intervention confirms previous experiences3 and our results could further the development of a prospective, randomized, controlled clinical trial.
In conclusion, adding CoPla to the treatment of critically ill patients with COVID-19 may be useful and it is both affordable and safe.











 nueva página del texto (beta)
nueva página del texto (beta)




