INTRODUCTION
Colorectal cancer is the third most frequent cancer and the second cause of death from cancer worldwide. Approximately 1.8 million new cases and 881,000 deaths from colorectal cancer are estimated for 20181. For 2017, it was estimated that 997 new cases of rectal cancer would be diagnosed in the community of Madrid2. Thanks to advances in research and treatment, the prognosis of patients with locally advanced rectal cancer (LARC) has improved greatly. In the 1980s, approximately 50% of patients with LARC relapsed, compared to only 10% currently. This improvement in relapse rates is due to the implementation of neoadjuvant chemoradiotherapy (nCRT) as standard treatment3,4, short-course radiotherapy (SCRT)5, and the surgical technique of total mesorectal excision6.
Nevertheless, despite improvement in local relapse rates, approximately 30% of patients will develop distant metastases, highlighting the importance of adjuvant chemotherapy (aCT) in the control of the metastatic disease. A meta-analysis7 of 21 randomized clinical trials, with a total of 9785 patients being affected with rectal carcinoma, identified a survival benefit for aCT. However, the conclusions of this meta-analysis were limited by the fact that the majority of patients included had not received nCRT. Several scores have been proposed for risk stratification of LARC patients8,9, most of which are based on analysis of the surgical tumor sample. The factors with high prognostic value include pathological complete response (pCR)10-12 and perineural invasion (PNI)13. In contrast, few risk scores include baseline patient characteristics, partially due to the limitations of diagnostic techniques, especially regarding lymph node involvement14.
Most official guidelines recommend aCT in the treatment of LARC, but there is no consensus on the specific regimen or the patient population. The National Comprehensive Cancer Network (NCCN) recommends the use of 5-fluorouracil (5-FU) plus oxaliplatin15, which is the standard adjuvant treatment in colon cancer16, while other guidelines, especially those from Northern Europe, recommend no adjuvant treatment17. The recommendations of other organizations, such as the European Society for Medical Oncology (ESMO) and the Spanish Society of Medical Oncology (SEOM), are based on risk stratification of patients and include follow-up with no aCT, 5-FU alone, or 5-FU plus oxaliplatin18-20.
To shed further light on the role of aCT in LARC and to explore the benefit of specific treatment regimens, we have analyzed a series of patients, all of whom had received nCRT followed by surgery with curative intent. We have compared outcomes and toxicity in patients receiving no aCT, adjuvant 5-FU, and adjuvant 5-FU plus oxaliplatin.
METHODS
Patients and study design
This was a retrospective observational study of 193 patients diagnosed and treated at the University Hospital of Fuenlabrada (Fuenlabrada, Spain) and the Alcorcon Foundation Hospital (Alcorcon, Spain) from January 2009 to December 2016. All patients included in the study had Stage II-III resectable rectal cancer (cT3-4 N+)21, with histologically confirmed adenocarcinoma. Diagnostic tests included a carcinoembryonic antigen (CEA) test, rectoscopy to measure the distance of the lower margin of the tumor from the anal verge, computed tomography (CT) of the thorax, abdomen, and pelvis to determine the extent of disease, and magnetic resonance imaging of the pelvis for local staging.
All patients were treated with nCRT followed by surgery with curative intent. After surgery, some patients received aCT.
The study was approved by the ethics committees of both participating centers and the Spanish Health Authorities (Agencia Española de Medicamentos y Productos Sanitarios).
nCRT and surgery
All patients received LCRT in 25 fractions of 1.8 Gy, for a total of 45 Gy, to the pelvis, followed by three sequential fractions of 1.8 Gy, for an additional 5.4 Gy, to the tumor and macroscopically suspect nodes. Intestinal extraction was done by extrinsic compression and bladder filling with the patient lying prone with a belly board. The prophylactic clinical target volumes included the mesorectum, posterior pelvic wall, and internal iliac nodes. The lower pelvis included tumors at < 6 cm from the anal verge, those involving the sphincter, and those subject to abdominoperineal resection (APR). The external iliac nodes were only included if other pelvic organs (uterus, bladder, vagina, prostate, and urethra) were involved. Inguinal nodes were only included for tumors affecting the external sphincter or the lower third of the vagina.
Concomitant neoadjuvant chemotherapy consisted of either 5-FU (225 mg/m2/day) by continuous infusion or oral capecitabine (825 mg/m2/12 h) during the 5 weeks of radiotherapy.
Surgery was performed 6-10 weeks after completion of neoadjuvant therapy by the surgical teams of the participating hospitals. Tumor samples were evaluated by the pathology departments of the participating hospitals. The pathological reports included the tumor pathological response (22, pathological TN stage, integrity of the mesorectum, circumferential margin involvement (< 1 mm), resected nodes, and lymphovascular invasion (LVI), and PNI23.
Adjuvant therapy
It was left at the discretion of the medical oncologist whether or not to administer adjuvant therapy and whether to administer 5-FU or 5-FU plus oxaliplatin. The comorbidity, the age of the patients, and the post-surgical toxicity were fundamental at the time of the decision of the adjuvant treatment. aCT consisted of either 14-day modified FOLFOX 6 (oxaliplatin 85 mg/m2, day 1; folic acid 200 mg/m2, days 1-2; bolus 5-FU 400 mg/m2, days 1-2; 5-FU 2400 mg/m2, and continuous infusion during 46 h) 8 cycles, 21-day CAPOX (oxaliplatin 130 mg/m2, day 1; capecitabine 1000 mg/m2/12 h, days 1-14) 5 cycles, or capecitabine (1000 mg/m2/12 h, days 1-14) 5 cycles.
Statistical analysis
Patients were included in the study by consecutive non-probability sampling. The primary endpoints were disease-free survival (DFS), calculated from surgery to disease progression or death, and overall survival (OS), calculated from surgery to death from any cause. It was estimated that 186 patients were needed, based on an 80% confidence level (type I error of 20%), power of 80%, expected survival of 50%, and a loss to follow-up of 5%.
Categorical variables were described by frequency or percentages and compared with the Chi-square or Fisher’s exact test. Quantitative variables were described by the mean and standard deviation (SD) or by median, 25th and 75th percentiles and were compared with the Student’s t-test. Normal distribution was checked with the Shapiro–Wilk test. Kaplan–Meier curves for DFS and OS were drawn and compared with the log-rank test. Univariate and multivariate regression analyses were performed to determine hazard ratios (HR) and 95% confidence intervals (CI) for DFS and OS. Variables identified as significant in the univariate analyses, or those that could be clinically relevant, were included in the multivariate analyses. All tests were two-sided, and significance was set at p ≤ 0.05. All analyses were performed with SPSS version 20.0.
RESULTS
Patient characteristics
We included 193 patients in the study. The median age was 63 years (range, 37 – 89). The distance of the tumor from the anal verge was <5 cm in 35.4% of cases, 5-8 cm in 32.4%, and 8-12 cm in 32.4%. Surgery was performed more than 8 weeks after completion of nCRT in 66.1 % of patients. A lower anterior resection (LAR) was performed in 73.4% of patients and an APR in 26.6%. A pCR was attained in 18.1% of patients. Twenty-nine patients (16%) received no adjuvant therapy, while the remaining 164 (83.9%) received either 5-FU (n = 74) or 5-FU plus oxaliplatin (n = 90). Characteristics were well-balanced among patients receiving aCT and those receiving observation (Table 1). Characteristics were also well-balanced among patients receiving 5-FU and those receiving 5-FU plus oxaliplatin, except for pathological stage and age, where the majority of patients receiving 5-FU alone had pathological Stage II (p = 0.001) and those receiving 5-FU plus oxaliplatin were younger (p = 0.002). Median cycles of FOLFOX were 7, median cycles of XELOX were 5, and 5 in the 5-FU arm.
Table 1 Characteristics of 193 patients included in the study
| Characteristic | No. adjuvant chemotherapy (n = 29) (%) | Adjuvant chemotherapy (n = 164) (%) | Adjuvant with 5-FU (n = 74) (%) | Adjuvant with oxaliplatin (n = 90) (%) |
|---|---|---|---|---|
| Age median (range) | 70 (51-88) | 63 (37-85) | 66.5 (37-86) | 60 (39-73) |
| Gender | ||||
| Male | 17 (58.6) | 109 (66.5) | 52 (70.3%) | 57 (63.3%) |
| Female | 12 (41.4) | 55 (33.5) | 22 (29.7) | 33 (36.7) |
| Clinical stage | ||||
| II | 2 (6.9) | 12 (7.3) | 7 (9.5) | 5 (5.6) |
| III | 27 (93.1) | 152 (92.7) | 67 (90.5) | 85 (94.4) |
| pCR | ||||
| Yes | 7 (24.5) | 28 (17.1) | 18 (24.3) | 10 (11.1) |
| No | 22(75.9) | 136 (82.9) | 56 (75.7) | 80 (88.9) |
| Pathological stage | ||||
| II | 18 (64.3) | 119 (17.1) | 63 (85.1) | 56 (62.2) |
| III | 10 (35.7) | 45 (27.4) | 11 (14.9) | 34 (37.8) |
| Not documented | 1 | |||
| LVI | ||||
| Yes | 7 (26.9) | 31 (19.9) | 10 (13.9) | 21 (25) |
| No | 19 (73.1) | 125 (80.1) | 62 (86.1) | 63 (75) |
| Not documented | 3 | 8 | 2 | 6 |
| PNI | ||||
| Yes | 11 (42.3) | 31 (20.3) | 12 (16.7) | 19 (23.5) |
| No | 15 (57.7) | 122 (79.7) | 60 (83.3) | 62 (76.5) |
| Not documented | 3 | 11 | 2 | 9 |
pCR, pathological complete response; PNI, perineural invasion; LVI, lymphovascular invasion.
Survival
With a mean follow-up of 89 months, 78.6% of patients remain disease-free. At the end of the follow-up, 25 patients died, 10 in the follow-up arm, and 15 in aCT arm. No patient was lost in the follow-up. A total of 41 recurrences were diagnosed.
Three patients relapsed with an exclusive local component, four with a mixed pattern with local and systemic failure and 33 with exclusive metastatic involvement, without relapse patterns being related to either the type of surgery or the systematic treatment employed. Mean DFS was 84.85 months (95% CI: 79-90) for patients receiving aCT, compared to 57.71 months (95% CI: 40-74) for those not receiving aCT (p < 0.001) (Fig. 1A). Mean OS was 92.7 months (95% CI: 88-97) and 66.18 months (95% CI: 51-81) (p < 0.001), respectively (Fig. 1B). When patients were classified according to aCT regimen, mean DFS was 83.65 months (95% CI: 76-91) for those receiving 5-FU alone and 82.97 months (95% CI: 75-90) for those receiving 5-FU plus oxaliplatin (p = 0.49) (Fig. 1C). Mean OS was 87 months (95% CI: 80-94) and 93.65 months (95% CI: 88-99) (p = 0.76), respectively (Fig. 1D).
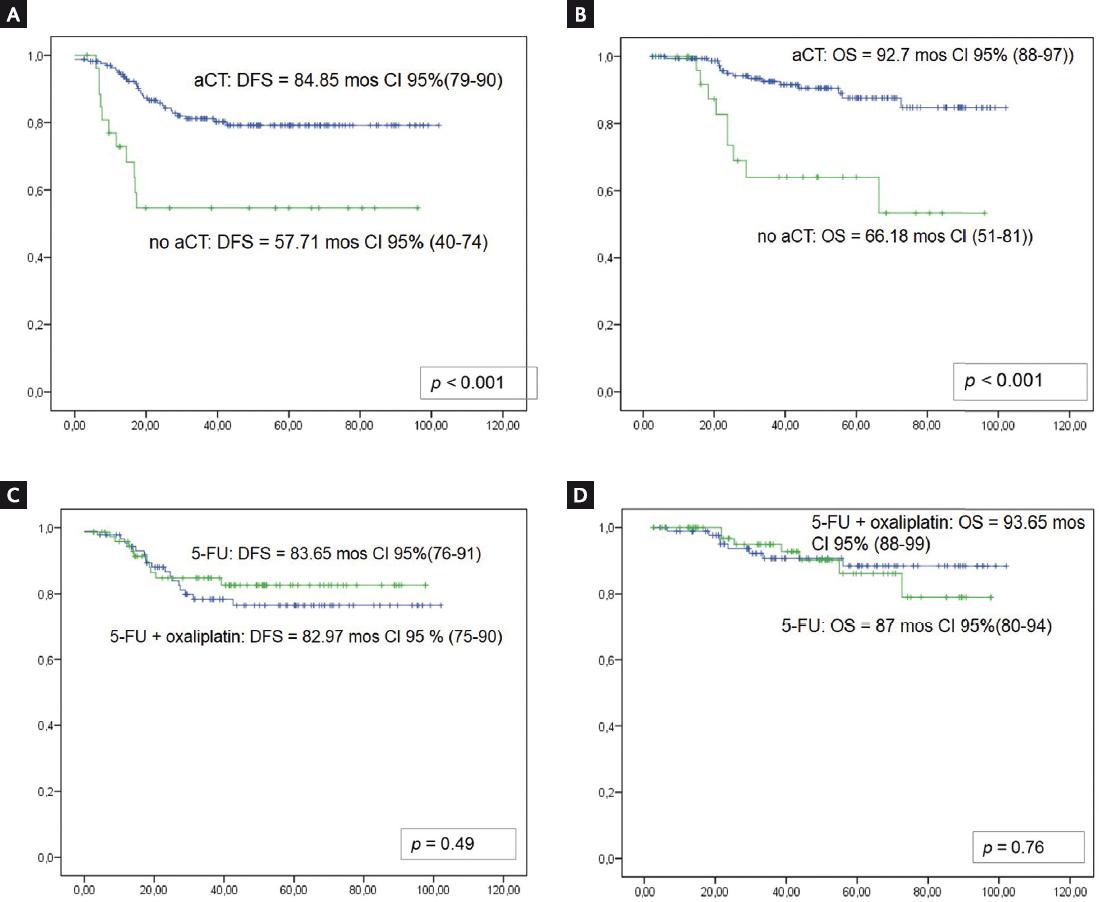
Figure 1 Kaplan–Meier curves for mean disease-free survival (DFS) and overall survival (OS). A: DFS for patients receiving adjuvant chemotherapy (aCT) (dotted line) and those not receiving aCT (solid line); B: OS for patients receiving adjuvant chemotherapy (aCT) (dotted line) and those not receiving aCT (solid line); C: DFS for patients receiving 5-FU (dotted line) and those receiving 5-FU plus oxaliplatin (solid line); D: OS for patients receiving 5-FU (dotted line) and those receiving 5-FU plus oxaliplatin (solid line).
In the univariate analysis of DFS, baseline CEA (p < 0.001), aCT (p < 0.001), pCR (p = 0.02), pathological stage (p = 0.001), LVI (p = 0.001), PNI (p < 0.001), and resection margin (p = 0.002) were associated with DFS (Table S1). The multivariate analysis identified aCT HR 0.21 (95% CI: 0.1-0.46) (p < 0.001), pCR HR 0.10 (95% CI: 0.01-0.46) (p = 0.03), and PNI HR 3.36 (95% CI: 1.7-6.5) (p < 0.001) as independent markers of DFS (Table S2).
Table S1 Univariate regression analyses for disease-free and overall survival
| Risk Factor | Disease-free survival | Overall survival | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Adjuvant chemotherapy | ||||
| Yes | 0.3 (0.15-0.62) | 0.001 | 0.22 (0.1-0.52) | <0.001 |
| No | Ref* | Ref* | ||
| Chemotherapy compliance | ||||
| Yes | Ref* | 0.56 | Ref* | 0.064 |
| No | 0.7 (0.2-2.3) | 3 (0.93-10) | ||
| Oxaliplatin | ||||
| Yes | 1.3 (0.6-2.7) | 0.48 | 0.85 (0.30-2.36) | 0.76 |
| No | Ref* | Ref* | ||
| Clinical stage | ||||
| II | Ref* | 0.43 | Ref* | 0.43 |
| III | 1.7 (0.4-7) | 2.2 (0.72-3.7) | ||
| Pathological stage | ||||
| II | Ref* | 0.001 | Ref* | 0.23 |
| III | 2.9 (1.5-5.3) | 1.63 (0.72-3.7) | ||
| Pathological complete response | ||||
| Yes | 0.87 (0.01-0.63) | 0.016 | Ref* | 0.16 |
| No | Ref* | 2.8 (0.66-11.98) | ||
| Lymphovascular invasion | ||||
| Yes | 3 (1.6-5.7) | 0.001 | 2.65 (1.1-6.3) | 0.03 |
| No | Ref* | Ref* | ||
| Perineural invasion | ||||
| Yes | 5.3 (2.8-10) | < 0.001 | 3.29 (1.42-7.6) | 0.005 |
| No | Ref* | Ref* | ||
| Resection margin | ||||
| Negative | Ref* | 0.002 | Ref* | 0.02 |
| Positive | 4.3 (1.6-11) | 4.10 (1.22-13) | ||
*Ref. is the baseline reference value for the calculation of HR in the Cox multivariate analysis. CI, confidence interval; HR, hazard ratio.
Table S2 Multivariate regression analyses for disease-free and overall survival, showing only significant values
| Risk Factor | Disease-free survival | Overall survival | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Adjuvant chemotherapy | ||||
| Yes | 0.21 (0.1-0.46) | < 0.001 | 0.21 (0.08-0.49) | < 0.001 |
| No | Ref* | Ref* | ||
| Pathological complete response | ||||
| Yes | 0.10 (0.01-0.75) | 0.03 | ||
| No | Ref* | 3 (0.93-10) | ||
| Perineural invasion | ||||
| Yes | 3.36 (1.7-6.5) | < 0.001 | 2.85 (1.22-6.6) | 0.015 |
| No | Ref* | |||
*Ref. is the baseline reference value for the calculation of HR in the Cox multivariate analysis.
CI, confidence interval; HR, hazard ratio.
In the univariate analysis of OS, baseline aCT (p < 0.001), LVI (p = 0.03), PNI (p = 0.005), and resection margin (p = 0.02) were associated with OS (Table 2). The multivariate analysis identified aCT HR 0.21 (95% CI: 0.08-0.49) (p < 0.001) and PNI HR 2.85 (95% CI: 1.22-6.6) (p = 0.02) as independent markers of OS (Table S2).
Table 2 Toxicities in patients receiving 5-FU alone or 5-FU plus oxaliplatin
| 5-FU | 5-FU plus oxaliplatin | p* | ||||
|---|---|---|---|---|---|---|
| Grade 1-2 | Grade 3-4 | Unknown | Grade 1-2 | Grade 3-4 | Unknown | <0.001 |
| 87.3% | 11.3% | 1.4% | 55.6% | 40% | 4.4% | |
*p-value calculated for differences in Grade 3-4 toxicities. 5-FU, 5-fluorouracil.
Toxicity
Episodes of Grade 3-4 toxicity were more frequent among patients receiving 5-FU plus oxaliplatin (40%) than those receiving 5-FU alone (11.3%) (p < 0.001) (Table 2). Treatment was discontinued in 17.6 % of patients receiving 5-FU plus oxaliplatin, compared to 11.3% of those receiving 5-FU alone. A dose reduction of oxaliplatin was required in 64.3% of patients.
DISCUSSION
The current standard of care for patients with LARC is nCRT or SCRT followed by total mesorectal excision. The option of neoadjuvant CT followed by CRT is also contemplated in patients with cT3-T4 N+ stages or with suspected circumferential margin affected. Most guidelines15,18,19 also recommend aCT, since distant metastases are the main cause of relapse in these patients. However, findings on the importance of aCT and on the optimal regimen to administer remain inconsistent4,24-26. We have examined the impact of aCT and compared two regimens in 193 patients with LARC, all of whom had received nCRT followed by surgery. Our findings indicate that aCT was an independent marker of longer DFS (HR 0.30; p 0.001) and OS (HR 0.22; p < 0.001). Moreover, while both DFS and OS were similar in patients receiving 5-FU alone and in those receiving 5-FU plus oxaliplatin, toxicity was greater in those receiving 5-FU plus oxaliplatin. In addition, the multivariate analysis of DFS identified pCR and PNI as independent markers of DFS – but not of OS.
There is little consensus on the use of aCT in LARC. The NCCN recommends 5-FU plus oxaliplatin for all patients, regardless of their post-surgical status15, while ESMO strongly recommends 5-FU plus oxaliplatin in patients with pathological Stage III disease but leaves it as an option in high-risk patients with pathological Stage II disease18. Guidelines from Northern European countries recommend follow-up only for all patients17. SEOM recommends personalized treatment based on risk stratification: for patients with pCR, follow-up only is an option; for those with negative nodes after nCRT, 5-FU is the aCT regimen of choice, although 5-FU plus oxaliplatin is an option; for patients with pT3-4 or N+, 5-FU plus oxaliplatin is recommended; finally, for frail patients or those with a life expectancy of < 5 years, follow-up with no aCT is recommended19.
To complicate this issue further, seven Phase III randomized trials, one Phase II randomized trial, and four meta-analyses have reported contradictory findings on the impact of aCT27. A review of five randomized trials comparing 5-FU aCT and observation or 5-FU-based and oxaliplatin-based aCT reported no benefit from aCT in either DFS or OS. The authors concluded that evidence did not support the use of aCT in patients with rectal cancer who had received nCRT followed by surgery27. A Cochrane meta-analysis of 21 randomized trials concluded that aCT reduced the risk of death by 17%7. However, since only one of the trials4 included patients who had received nCRT – now considered the standard treatment – these results cannot be considered conclusive. It became necessary to develop studies investigating the role of aCT after standard neoadjuvant treatment. A more recent meta-analysis looked at randomized trials that included only patients who had received nCRT. For five trials comparing aCT versus observation, the meta-analysis found no benefit for aCT, while in four trials comparing 5-FU with 5-FU plus oxaliplatin, the pooled difference in DFS was not statistically significant28. In colon cancer, in contrast, aCT has been shown to confer a survival benefit, leading some authors to suggest that the lack of benefit in LARC may be due to the longer interval between surgery and starting aCT in LARC compared to colon cancer. For every 4 weeks of delay in starting aCT after surgery, there is a 14% increase in the risk of death7.
Toxicity associated with aCT is common. The MOSAIC trial reported oxaliplatin-related Grade 3 toxicities in 12.5% of patients receiving oxaliplatin, compared to 0.2% in those receiving 5-FU29. Other studies have reported toxicities of 15-30%, with a need for treatment interruption30. In line with these studies, we found a higher frequency of Grade 3-4 toxicities in patients receiving oxaliplatin than in those receiving 5-FU alone. The need for treatment discontinuation in our study (17.6% of patients receiving 5-FU plus oxaliplatin) was also similar to that reported previously. pCR10-12 and PNI are both well-known prognostic markers in rectal cancer. In the present study, in addition to aCT, the multivariate analysis identified pCR as a marker of longer DFS (HR 0.10; p = 0.03) but not of OS, and PNI as a marker of shorter DFS (HR 3.36; p < 0.001) and OS (HR 2.85; p = 0.02).
Given this scenario of conflicting reports, our study can provide some useful indications as to the benefit of aCT in LARC. While our conclusions are necessarily limited by its retrospective nature, its relatively small sample size and the limited number of cases in clinical Stage II, our study has the advantage of a homogeneous cohort of patients, all of whom received nCRT followed by surgery. Our findings lead us to recommend the use of aCT in LARC; however, the toxicity associated with the use of oxaliplatin – with no corresponding increase in survival benefit compared to 5-FU alone – suggests that 5-FU may be a better option for these patients. Nevertheless, our data are preliminary and the number of events is low; therefore, we will have to wait for more conclusive results.
SUPPLEMENTARY DATA
Supplementary data are available at Revista de Investigación Clínica online (www.clinicalandtranslationalinvestigation.com). These data are provided by the corresponding author and published online for the benefit of the reader. The contents of supplementary data are the sole responsibility of the authors.











 nova página do texto(beta)
nova página do texto(beta)


