INTRODUCTION
As one of the most prevalent cancers, esophageal cancer (EC) is the 6th leading cause of cancer mortality worldwide1,2. Esophageal squamous cell carcinoma (ESCC) is the most common pathological type of EC in China3. Although radical surgical resection is the standard and effective treatment, the prognosis in patients with EC remains poor4,5. To predict the prognosis of EC; therefore, it is increasingly urgent and important to explore more useful and effective prognostic biomarkers.
It has been reported that fibrinogen (Fib), a major protein in the blood clotting process, is associated with cancer6. Although recent studies have reported that plasma Fib was related to cancer progression and prognosis in various cancers7-9, the role of plasma Fib in EC remains controversial10-12. Recently, two meta-analyses revealed that plasma Fib was a prognostic indicator in patients with EC13,14. As a nutritional factor, albumin (ALB) reflected the nutritional status in a variety of cancers, including patients with EC15,16. A new prognostic index in recent years was proposed with fibrinogen-to-albumin ratio (FAR) for patients with ESCC17.
Prealbumin (PALB) is another important factor for nutritional status, which is more sensitive to malnutrition than ALB18,19. According to the above theoretical basis; therefore, we hypothesized that PALB could substitute for ALB to combine with Fib to construct a new index: fibrinogen to prealbumin ratio (FPR). A novel prognostic index with FPR was initially proposed to predict the overall survival (OS) and cancer-specific survival (CSS) in patients with resectable ESCC patients.
METHODS
Patients
Between January 2006 and December 2010, a retrospective study, including 372 patients with resectable ESCC was conducted in our department. Inclusion criteria were: (1) patients with ESCC in TNM Stage I-III confirmed by histopathology; (2) patients with curative surgical treatment performed without any neoadjuvant treatment; (3) patients without any form of inflammatory diseases; and (4) pre-operative Fib, ALB, PALB, neutrophil count, and lymphocyte count obtained 1 week before surgery. Informed consent regarding the collection of specimens was signed by each patient before surgery. The Zhejiang Cancer Hospital Ethics Committee approved the current study in June 2018 (IRB2018-130). All patients in this study participated in accordance with the principles embodied in the Declaration of Helsinki.
Treatment and follow-up
All of the patients underwent radical esophagectomy with lymphadenectomy. The standard esophagectomy, including the Ivor-Lewis and McKeown procedures, was determined by the tumor location20,21. The two-field was the main method of lymphadenectomy used22. Patients with any neoadjuvant treatment were excluded from the study. Post-operative adjuvant therapy was not mandatory because adjuvant therapy was still controversial during that period (2006-2010). Cisplatin and 5-fluorouracil were the most frequent drugs for adjuvant chemotherapy. A total dose of 50-60 Gy for postoperative radiotherapy was delivered in 25-30 fractions (2.0 Gy/fraction). The follow-up was performed at regular intervals in all patients (every 3 months for the first 2 years, every 6 months for the next 3 years, then annually). The last follow-up date was June 2014.
Data collection and measurement
The clinical characteristics, including gender, age, vessel invasion, tumor location, differentiation, tumor length, TNM stage, PALB, ALB, Fib, neutrophil count, and lymphocyte count, were collected. The TNM stage was in accordance with the 7th AJCC/UICC TNM staging system23. The levels of Fib, PALB, ALB, neutrophil count, and lymphocyte count were obtained within 1 week before surgery. Neutrophil and lymphocyte counts were performed by automated blood cell counter (Sysmex XE-2100, Kobe, Japan). The level of Fib was determined by automatic coagulation analyzer (Sysmex CA-7000, Kobe, Japan). Levels of ALB and PALB were measured by latex-enhanced homogeneous immunoassay (Hitachi 917; Skill, Munich, Germany).
Statistical analysis
The best cutoff points for NLR, Fib, PALB, ALB, FAR, and FPR were selected by receiver operating characteristic (ROC) curves according to the CSS status (alive or death). The Chi-squared test was used to analyze the categorical variables of characteristics grouped by FPR. CSS and OS were evaluated with Cox regression analyses (forward stepwise regression). Two models for Cox analyses (one for categorical variables and the other for continuous variables) were utilized to determine hazard ratio (HR) with 95% confidence interval (CI) for CSS and OS24-26. The areas under the curve (AUC) for NLR, Fib, FAR, FPR, ALB, and PALB were calculated and compared. A nomogram model was performed with R 3.6.0 software. MedCalc 15.2 (MedCalc Software bvba, Ostend, Belgium) and SPSS 20.0 (SPSS Inc., Chicago, IL, USA) were performed to analyze the statistical analyses.
RESULTS
Patient characteristics
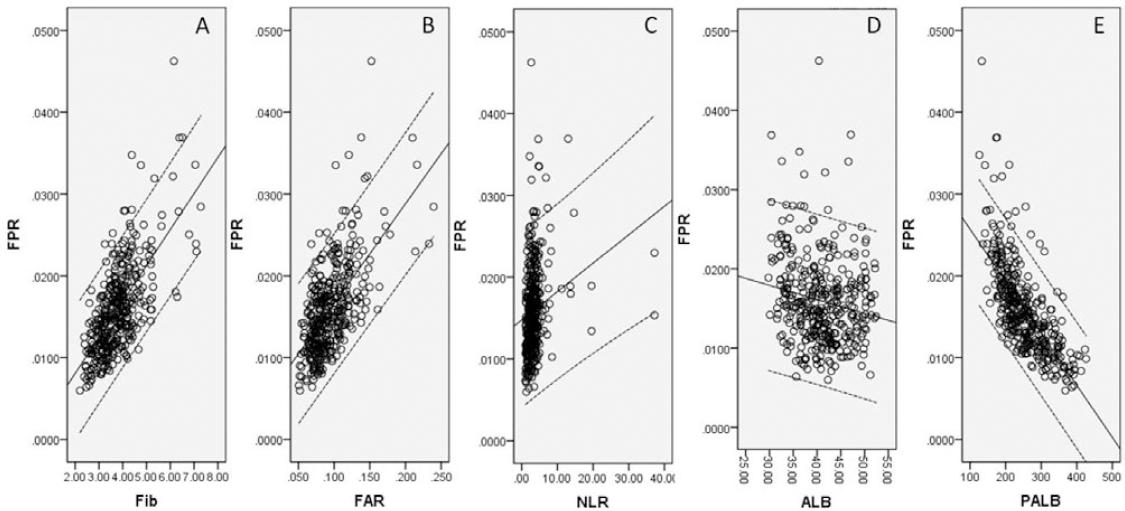
The clinicopathologic characteristics are shown in table 1. According to clinical criteria, a total of 115 patients (30.9%) received post-operative adjuvant radiotherapy and/or chemotherapy. The best cutoff values for Fib, PALB, ALB, FAR, FPR, and NLR were 3.95 g/L, 235.0 mg/L, 42.0 g/L, 0.1, 0.014, and 3.76, respectively (Fig. 1). According to the cutoff level, patients were then divided into two groups (FPR ≤ 0.014 and FPR > 0.014) (Table 1). In our study, the FPR was statistically significantly associated with TNM stage, ALB, PALB, NLR, Fib, and FAR. Pearson’s correlation analyses revealed that FPR was positively correlated with Fib (r = 0.673, p < 0.001), FAR (r = 0.622, p < 0.001), and NLR (r = 0.206, p < 0.001), but was negatively correlated with ALB (r = −0.175, p = 0.001) and PALB (r = −0.713, p < 0.001) (Fig. 2).
Table 1 Comparison of the clinical characteristics according to fibrinogen/pre-albumin ratio in esophageal squamous cell carcinoma patients
| Cases (n, %) | FPR | p-value | ||
|---|---|---|---|---|
| Low (n, %) | High (n, %) | |||
| Age (years) | ||||
| ≤60 | 210 (56.5) | 78 (52.0) | 132 (59.5) | 0.155 |
| >60 | 162 (43.5) | 72 (48.0) | 90 (40.5) | |
| Gender | 0.711 | |||
| Female | 45 (12.1) | 17 (11.3) | 28 (12.6) | |
| Male | 327 (87.9) | 133 (88.7) | 194 (87.4) | |
| Tumor length (cm) | ||||
| ≤3.0 | 106 (28.5) | 48 (32.0) | 58 (26.1) | 0.218 |
| >3.0 | 266 (71.5) | 102 (68.0) | 164 (73.9) | |
| Tumor location | 0.901 | |||
| Upper | 25 (6.7) | 9 (6.0) | 16 (7.2) | |
| Middle | 172 (46.2) | 70 (46.7) | 102 (45.9) | |
| Lower | 175 (47.1) | 71 (47.3) | 104 (46.9) | |
| Vessel invasion | 0.136 | |||
| Negative | 312 (83.9) | 131 (87.3) | 181 (81.5) | |
| Positive | 60 (16.1) | 19 (12.7) | 41 (18.5) | |
| Perineural invasion | 0.076 | |||
| Negative | 293 (78.8) | 125 (83.3) | 168 (75.7) | |
| Positive | 79 (21.2) | 25 (16.7) | 54 (24.3) | |
| Differentiation | 0.064 | |||
| Well | 52 (14.0) | 22 (14.7) | 30 (13.5) | |
| Moderate | 246 (66.1) | 107 (71.3) | 139 (62.6) | |
| Poor | 74 (19.9) | 21 (14.0) | 53 (23.9) | |
| TNM stage | 0.001 | |||
| I | 94 (25.3) | 47 (31.3) | 47 (21.2) | |
| II | 119 (32.0) | 56 (37.4) | 63 (28.4) | |
| III | 159 (42.7) | 47 (31.3) | 112 (50.4) | |
| ALB (g/L) | 40.94 ± 5.46 | 41.95 ± 4.69 | 40.26 ± 5.84 | 0.002 |
| ≤42.0 | 219 (58.9) | 79 (52.7) | 140 (63.1) | 0.046 |
| >42.0 | 153 (41.1) | 71 (47.3) | 82 (36.9) | |
| PALB (mg/L) | 252.9 ± 62.0 | 301.3 ± 53.0 | 222.3 ± 43.5 | < 0.001 |
| ≤235.0 | 175 (47.0) | 19 (12.7) | 156 (70.3) | < 0.001 |
| >235.0 | 197 (53.0) | 131 (87.3) | 66 (29.7) | |
| NLR | 3.48 ± 3.28 | 2.79 ± 1.85 | 3.95 ± 3.90 | < 0.001 |
| ≤3.76 | 266 (71.5) | 124 (82.7) | 142 (64.0) | < 0.001 |
| >3.76 | 106 (28.5) | 26 (17.3) | 80 (36.0) | |
| Fib (g/L) | 3.80 ± 0.85 | 3.31 ± 0.55 | 4.13 ± 0.85 | < 0.001 |
| ≤3.95 | 232 (62.4) | 125 (83.3) | 107 (48.2) | < 0.001 |
| >3.95 | 140 (37.6) | 25 (16.7) | 115 (51.8) | |
| FAR | 0.095 ± 0.028 | 0.080 ± 0.015 | 0.105 ± 0.030 | < 0.001 |
| ≤0.1 | 239 (64.2) | 132 (88.0) | 107 (48.2) | < 0.001 |
| >0.1 | 133 (35.8) | 18 (12.0) | 115 (51.8) | |
ESCC: esophageal squamous cell carcinoma, ALB: albumin, PALB: prealbumin, TNM: tumor node metastasis, NLR: neutrophil-to-lymphocyte ratio, Fib: fibrinogen, FAR: fibrinogen-to-albumin ratio, FPR: fibrinogen-to-prealbumin ratio.
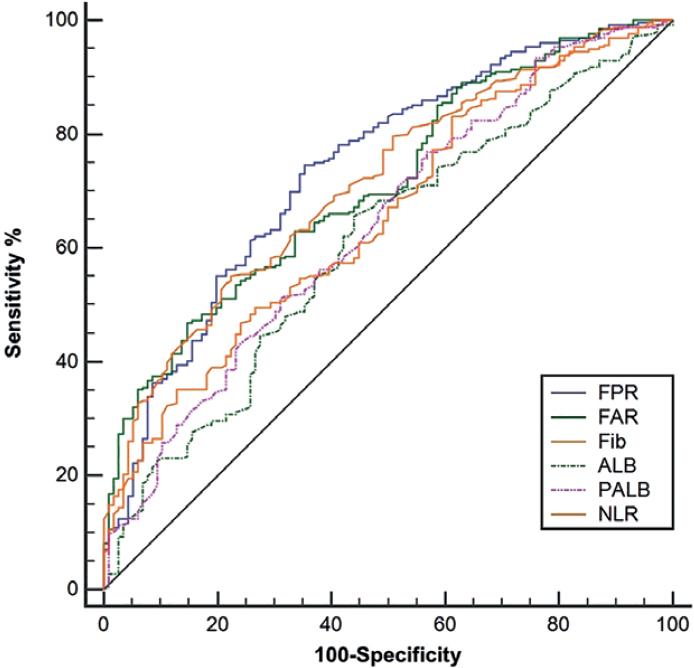
Figure 1 ROC curves analyses regarding the best cutoff values and AUC areas. The best cutoff values for Fib, ALB, PALB, FAR, FPR, and NLR were 3.95 g/L, 42.0 g/L, 235.0 mg/L, 0.1, 0.014, and 3.76, respectively. The AUC areas were 0.739, 0.711, 0.712, 0.653, 0.608, and 0.640 for FPR, FAR, Fib, NLR, ALB, and PALB, respectively.
CSS and OS analysis
At the last follow-up time, 261 (70.2%) of the 372 patients had died. The median follow-up time and survival time were 73 months and 32 months, respectively. Patients with lower levels of FPR (≤ 0.014) had better CSS (50.7% vs. 18.0%, p < 0.001) and OS (48.0% vs. 17.6%, p < 0.001) (Fig. 3A and B). In subgroup analyses based on the TNM stage (TNM I, TNM II, and TNM III), we found that FPR was also significantly related to CSS (Fig. 3C-E) and OS (Fig. 3F-H). Using categorization in multiple groups (instead of two) would help to define a biological gradient; therefore, we divided FPR into three groups (≤ 0.01, 0.01-0.02, and > 0.02). The results also revealed that FPR (divided into three groups) was also significantly associated with CSS (Fig. 3I) and OS (Fig. 3J).
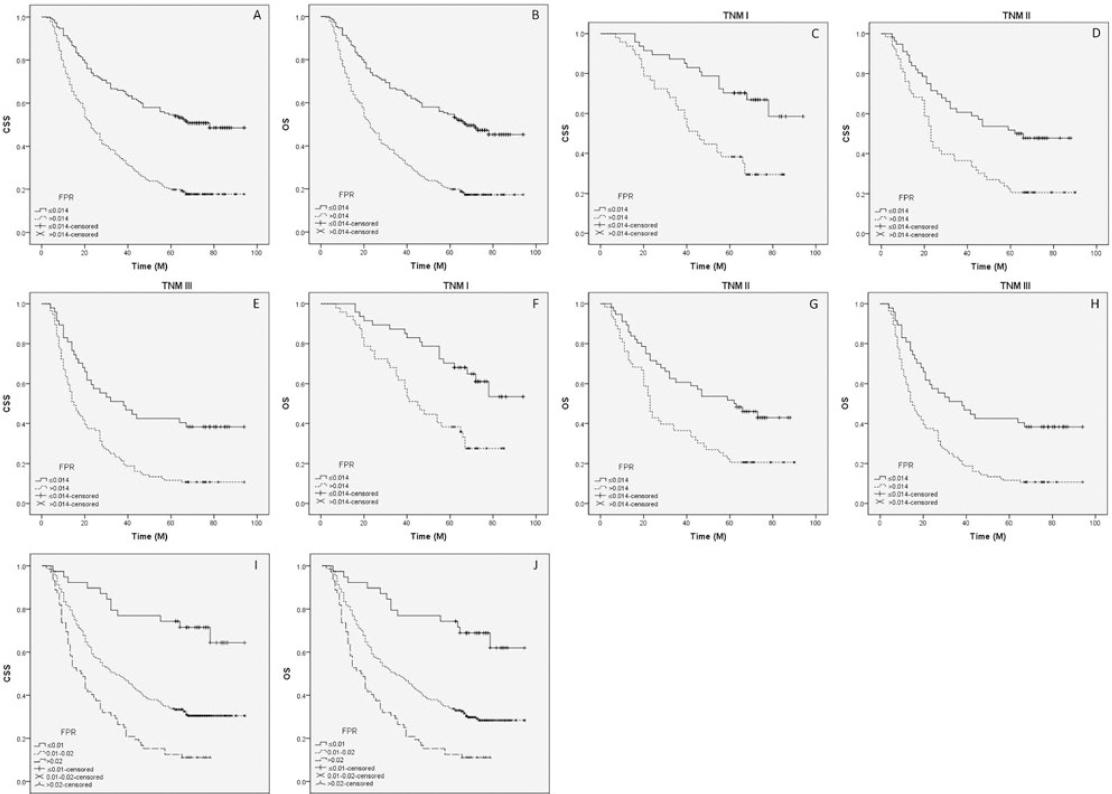
Figure 3 Kaplan–Meier CSS and OS curves. Kaplan–Meier curves regarding CSS (50.7% vs. 18.0%, p < 0.001; A) and OS (48.0% vs. 17.6%, p < 0.001; B) grouped by FPR. Kaplan-Meier curves regarding CSS (TNM I: 31.9% vs. 66.0%, p < 0.001, C; TNM II: 20.6% vs. 48.2%, p = 0.001, D; TNM III: 10.7% vs. 38.3%, p < 0.001, E) and OS (TNM I: 29.8% vs. 61.7%, p = 0.001, F; TNM II: 20.6% vs. 44.6%, p = 0.002, G; TNM III: 10.7% vs. 38.3%, p < 0.001, H) in subgroup analyses based on TNM stage. Kaplan- Meier curves regarding CSS (69.2% vs. 31.0% vs.11.1%, p < 0.001; I) and OS (66.7% vs. 29.5% vs.11.1%, p < 0.001; J) grouped by FPR divided into three groups (≤ 0.01, 0.01-0.02 and > 0.02).
Cox regression analysis
Two models for Cox analyses (one for categorical variables and the other for continuous variables) were used. Both univariate analyses, using categorical and continuous variables, indicated that tumor length, vessel invasion, perineural invasion, TNM stage, NLR, Fib, ALB, PALB, FAR, and FPR were significantly associated with CSS and OS (Table S1). Multivariate Cox analyses (categorical and continuous) demonstrated that FPR was an independent prognostic factor in CSS (categorical: HR: 2.014, 95% CI: 1.504-2.697, p < 0.001; continuous per 0.01: HR: 1.438, 95% CI: 1.154-1.793, p = 0.001) and OS (categorical: HR: 1.964, 95% CI: 1.475-2.617, p < 0.001; continuous per 0.01: HR: 1.429, 95% CI: 1.146-1.781, p = 0.002) (Table S2).
ROC analysis
The AUC areas were 0.739, 0.711, 0.712, 0.653, 0.608, and 0.640 for FPR, FAR, Fib, NLR, ALB, and PALB, respectively (Fig. 1). Comparison of AUC areas regarding the FPR, FAR, Fib, NLR, ALB, and PALB in ESCC is shown in Table S3.
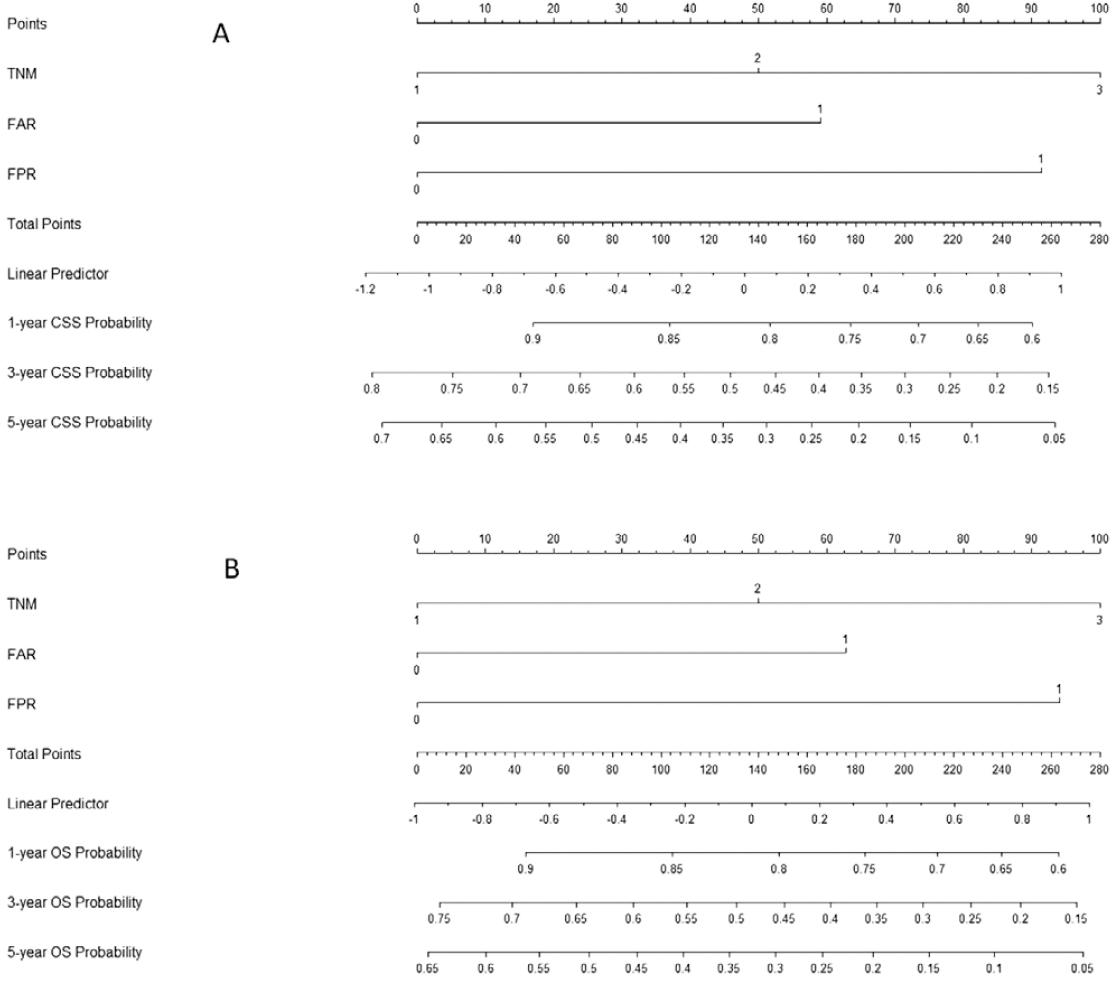
Nomogram analysis
A nomogram, using three independent prognostic factors (TNM, FAR, and FPR) in multivariate analyses, was used to predict the CSS and OS (Fig. 4). The 1-, 3-, and 5-year CSS and OS probability of ESCC patients could be predicted according to this nomogram model.
DISCUSSION
As far as we know, this is the first study in ESCC to indicate the prognostic value of FPR (Fib/PALB ratio). Our study revealed that pre-operative FPR serves as an independent prognostic factor for CSS (HR: 2.014, 95% CI: 1.504-2.697, P<0.001) and OS (HR: 1.964, 95% CI: 1.475-2.617, p < 0.001). Moreover, we first performed a nomogram using TNM, FAR, and FPR to predict the 1-, 3-, and 5-years CSS and OS probability in patients with ESCC.
The prognostic role of plasma Fib in EC is still controversial. Wakatsuki et al.10 and Takeuchi et al.11 demonstrated that plasma Fib was a useful independent predictive indicator for prognosis in ESCC. Conversely, Li et al.12 revealed that Fib was not associated with the OS of ESCC. Recently, two meta-analyses revealed that plasma Fib was a prognostic indicator in patients with EC13,14. However, the present study revealed that Fib was not associated with CSS or OS in ESCC patients.
The prognostic value of ALB, as a nutritional indicator, has been confirmed in a variety of cancers, including patients with EC15,16. A new prognostic index in recent years was proposed with FAR for patients with ESCC17. PALB, as another important nutritional indicator, is more sensitive to malnutrition than ALB18,19. However, no study regarding the prognostic role of FPR has been assessed so far in ESCC patients. Moreover, the prognostic role in patients with ESCC between FPR (Fib/PALB) and FAR (Fib/ALB) is still unknown. In the current study, patients with lower levels of FPR (≤ 0.014) had better CSS (50.7% vs. 18.0%, p < 0.001) and OS (48.0% vs. 17.6%, p < 0.001). FPR serves as an independent prognostic factor for CSS (HR: 2.014, 95% CI: 1.504-2.697, p < 0.001) and OS (HR: 1.964, 95% CI: 1.475-2.617, p < 0.001).
As we know, Fib, PALB, and ALB are all routinely tested blood indexes in daily clinical practice. In the present study, we have first explored the prognostic value of FPR comparing with Fib and FAR in ESCC. In our results, the AUC area was 0.739, 0.711, 0.712, 0.653, 0.608, and 0.640 for FPR, FAR, Fib, NLR, ALB, and PALB, respectively. Moreover, patients with lower levels of FPR (≤ 0.014) were associated with better CSS (p < 0.001).
Pre-operative neoadjuvant treatment (chemotherapy and/or radiotherapy) followed by surgery improves survival in several randomized control trials. The OEO2 trial in operable EC patients with pre-operative chemotherapy revealed that pre-operative chemotherapy followed by surgery prolonged the DFS (HR = 0.82, 95% CI: 0.71-0.95, p = 0.003) and OS (HR = 0.84, 95% CI: 0.72-0.98, p = 0.03)27. The CROSS trial including 366 patients with esophageal or junctional cancer also demonstrated that neoadjuvant chemoradiotherapy followed by surgery improved DFS (HR = 0.498, 95% CI: 0.357-0.693, p < 0.001) and OS (HR = 0.657, 95% CI: 0.495-0.871, p = 0.003)28. However, the French trial with 195 EC patients in TNM Stages I and II (FFCD 9901) revealed that pre-operative chemoradiotherapy followed by surgery did not improve DFS (HR = 0.92, 95% CI: 0.66-1.30, p = 0.648) or OS (HR = 0.99, 95% CI: 0.69-1.40, p = 0.94)29. The survival benefit of neoadjuvant chemoradiotherapy in patients with EC was confirmed in two meta-analyses30,31. In the current study, however, patients with any neoadjuvant treatment were excluded due to two reasons. On the one hand, pre-operative neoadjuvant therapy was controversial during that period (2006-2010). On the other hand, patients with pre-operative therapy might have a side effect on the levels of blood indexes.
There is no general consensus on post-operative adjuvant therapy in patients with EC. A randomized, controlled Phase III trial (JCOG9204) including 242 patients with ESCC revealed that surgery plus post-operative adjuvant chemotherapy showed better 5-year DFS (55% vs. 45%, p = 0.037), but not for 5-year OS (61% vs. 52%, p = 0.13)32. In another Japanese randomized trial (JCOG9907) for patients with Stage II/III ESCC, the results revealed that pre-operative chemotherapy followed by surgery improved OS (HR = 0.973, 95% CI: 0.54-0.99, p = 0.04) compared to post-operative adjuvant chemotherapy33. In the current study, according to clinical criteria, a total of 115 patients (30.9%) received post-operative adjuvant radiotherapy and/or chemotherapy. However, there were no statistical differences between patients with and without adjuvant treatment in CSS (28.7% vs. 32.3%, p = 0.129) or OS (27.8% vs. 30.7%, p = 0.152). The main reason was that patients with TNM I stage were included in the current study.
It is generally recognized that blood indexes including Fib and PALB may be influenced by various conditions. Therefore, the role of FPR would be more reliable. In the current study; moreover, we found positive correlations between FPR and NLR and negative correlations between FPR and ALB/PALB. Therefore, FPR is a combined indicator which can reflect a mixed prognostic role.
Some potential limitations of the current study should be mentioned. First, because this is a retrospective study in a single-center, potential selection bias cannot be excluded entirely. Second, patients with pre-operative treatment were excluded from the study, which might have influenced the results for patients with ESCC29,34. Third, due to the lack of a prospective study design, the results of our study should be validated in more large-sample trials in the future. Any prognostic index must consider the AJCC necessary criteria for developing prognostic models. The AJCC committee proposed 13 inclusion and three exclusion criteria for a risk model35. However, we must acknowledge that our prognostic model was only developed, but not validated in this study. Therefore, the results of our study should be validated in future studies.
Our study indicated that FPR (cutoff point: 0.014) was an independent prognostic factor in patients with resectable ESCC. Patients with lower levels of FPR (≤ 0.014) had better CSS and OS than patients with higher levels of FPR (> 0.014).











 nueva página del texto (beta)
nueva página del texto (beta)