INTRODUCTION
According to the international agency for research on cancer, in 2015, approximately 414,772 new cases of non-Hodgkin lymphoma (NHL) were diagnosed worldwide1. Diffuse large B-cell lymphoma (DLBCL) is the most frequent type of NHL. Although this disease is potentially curable2, the outcome of patients may vary according to the primary site involved. Before the WHO classification of lymphoproliferative disorders3, involvement of the central nervous system (CNS) was the most frequent extranodal manifestation; however, this site constitutes an independent entity4. Recently, some authors have reported that primary extranodal (PE) involvement may be associated with a longer survival in comparison with patients with only nodal involvement in a study of patients with DLBCL of the head and neck5. In fact, primary breast, primary bone, and primary craniofacial DLBCL are extranodal manifestations of DLBCL, and most studies agree with the low incidence in these sites. As a primary manifestation, primary breast lymphoma represents up to 0.5% of all NHL and approximately 2% of extranodal presentations6,7; primary bone DLBCL constitutes approximately 4-5% of extranodal lymphomas and approximately 3% of all malignant bone tumors8,9; and craniofacial DLBCL accounts for approximately 1% of all lymphomas and 2-12% of extranodal lymphomas10,11.
Because of the rarity of these manifestations, most of the studies are limited and, as far as we know, there is no available information regarding extranodal DLBCL (N-DLBCL) (extranodal-DLBCL) in Latin American countries. The objective of this work was to study at a single academic cancer center, the relative frequency of the location, clinical characteristics, clinical response, and survival of patients with these primary sites and compares them with those of the classical primary N-DLBCL.
METHODS
Patients
This is a retrospective cohort study of patients with PE-DLBCL or (N-DLBCL) treated at the national cancer institute (Instituto Nacional de Cancerología) in Mexico City from January 2011 to June 2017. Patients had to be older than 18 years, have a histopathological diagnosis of DLBCL, and should have been treated in our institution. Cohorts were defined according to the extranodal locations. Exposures were the clinical characteristics of cases, and outcome measures were the response to treatment, progression-free survival (PFS), and overall survival (OS).
The clinical characteristics analyzed were age, sex, body mass index, comorbidities (i.e., presence of diabetes mellitus, systemic arterial hypertension, cardiopathy, viral hepatitis, and HIV status), presence of B symptoms, bulky disease, clinical stage defined by the Lugano classification, number of extranodal sites, and performance status as defined by the ECOG scale12. The basal blood cytology and biochemistry data included were blood hemoglobin; leukocyte, lymphocyte, and platelet counts; serum albumin; prognostic nutritional index; lactic dehydrogenase (LDH); and beta-2 microglobulin levels.
Histopathological classification was performed by the Hans nomogram and was based on the expression of CD10, BCL6, and MUM1, as previously described13. Briefly, samples expressing CD10 (+) or CD10 (−), BCL6 (+), and MUM1 (−) were defined as of germinal center origin (GC); and samples expressing CD10 (−), BCL6 (−) or CD10 (−), BCL6 (+), and MUM1 (+) were considered as non-GC origin. Clinical staging was performed by standard methods, including positron emission tomography (PET)-computed tomography (CT), bone marrow biopsy and lumbar puncture, if clinically indicated.
Cohorts were defined according to the primary extranodal (breast, bone, craniofacial, gastrointestinal, and primary testicular) or nodal location. Cohort A corresponded to primary breast DLBCL and was defined according to Wiseman and Liao criteria14 as follows: (a) the breast was the site of clinical presentation; (b) no prior history of lymphoma or evidence of widespread disease; (c) lymphoma in close association to breast tissue on pathological assessment; and (d) ipsilateral regional lymph nodes may be involved. Cohort B corresponded to primary bone DLBCL and included (1) patients with lymphoma only on a bone site with or without regional lymph-node involvement; and (2) lymphoma with multiple bones involved but no visceral or lymph node involvement. Cohort C had primary craniofacial DLBCL and was defined by the presence of a primary craniofacial involvement without regional nodal involvement and no other extranodal site involved. Cohort D corresponded to primary gastrointestinal site and included patients with either only gastrointestinal infiltration or gastrointestinal and regional lymph nodes. Cohort E included patients with primary testicular lymphoma; those with extensive lymph node disease and also testicular infiltration were classified as nodal, clinical Stage IV. Cohort F corresponded to the classical nodal involvement of DLBCL. Primary mediastinal and primary central nervous DLBCL were not included since they are considered as independent entities within the 2016 WHO lymphoproliferative disorders classification3.
Treatment
Treatment schemes included the following: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (375 mg/m2 rituximab, 750 mg/m2 cyclophosphamide, 50 mg/m2 doxorubicin, 1.4 mg/m2 vincristine (total maximal dose: 2 mg), and 100 mg/day/5 days prednisone); Dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, rituximab (375 mg/m2 rituximab; 50 mg/m2 etoposide, daily in 96 h continuous infusion; 10 mg/m2 doxorubicin, daily in 96 h continuous infusion; 0.4 mg/m2 vincristine, daily in 96 h continuous infusion, and 750 mg/m2 cyclophosphamide on day 5)15. Furthermore, radiotherapy (dose and site) was added when clinically indicated. The response to treatment was evaluated using standard international criteria. For patients in whom PET/CT was performed before and after treatment, Deauville criteria were used16. In cases with increased blood-glucose levels (>170 mg/dL), which contraindicated a PET/CT, only a CT was performed, and the response was evaluated by standard CHESON criteria17.
Statistical Analysis
After descriptive analysis, comparison between groups was performed by employing one-way ANOVA or a Chi-square test, as required. PFS was defined from the date of diagnosis until the date of relapse, and OS was defined since diagnosis until the date of last follow-up or death. Survival curves were constructed employing the KaplanMeier method, and differences in survival between groups were determined with the Log-Rank test. The Cox model was used to identify the clinical, biochemical, or histopathological variables predicting OS. Analyses were performed using SPSS, version 24.0 (IBM Corp., Armonk, NY, USA), and probability values of 0.05 or lower were considered significant.
RESULTS
Clinical and Pathological Characteristics
We included 637 patients during the study period. From them, only 51 (8.0%) were considered as PE-DLBCL. Cohorts A, B, C, D, E, and F included 6, 5, 12, 25, 3, and 586 patients, respectively. Demographic and clinical characteristics of patients are listed in table 1.
Table 1 Demographic and clinical characteristics at diagnosis, by cohort
| Variables n (%) | Cohorts | ||||||
|---|---|---|---|---|---|---|---|
| A Breast | B Bone | C Craniofacial | D Gastrointestinal | E Testicular | F Nodal | p-value | |
| 6 (0.94) | 5 (0.78) | 12 (1.8) | 25 (3.9) | 3 (0.46) | 586 (91.7) | 0.256 | |
| Female/male | 6:0 | 2:3 | 5:7 | 14:11 | 0:3 | 303:283 | |
| Age (mean ± SD) | 53.3 ± 21.2 | 46.8 ± 18.42 | 67.924 ± 19.68 | 55.72 ± 13.5 | 64.23 ± 6.7 | 57.22 ± 15.52 | <0.01 |
| BMI (mean ± SD) | 24.7 ± 3.50 | 23.29 ± 4.50 | 24.2 ± 3.87 | 26.65 ± 4.5 | 27.3 ± 2.6 | 24.45 ± 5.90 | 0.864 |
| Diabetes | 0 | 1 | 3 | 3 | 0 | 77 (13.13) | 0.664 |
| Systemic arterial hypertension | 3 (50) | 0 (0) | 2 (16.66) | 4 (16) | 1 (33.3) | 107 (18.25) | 0.487 |
| ECOG | |||||||
| 0 | 2 (33.33) | 1 (20) | 3 (25) | 3 (12) | 2 (66.6) | 89 (15.19) | 0.932 |
| 1 | 4 (66.67) | | 6 (50) | 14 (56) | 1 (33.3) | 282 (48.12) | |
| 2 | | 1 (20) | 2 (16.67) | 6 (24) | | 125 (21.33) | |
| 3 | | 3 (60) | 1 (8.33) | 2 (8) | | 73 (12.45) | |
| 4 | | | | | | 17 (2.91) | |
| B symptoms | 2 (33.33) | 1 (20) | 3 (25) | 18 (72) | 3 (100) | 379 (64.67) | 0.572 |
| LDH elevated | 3 (50) | 2 (40) | 2 (16.67) | 18 (72) | 3 (100) | 359 (61.26) | 0.028 |
| HIV-positive | 0 | 0 | 0 | 2 (8) | 0 | 44 (7.50) | 0.002 |
| HBV/HCV positive | 0 | 0 | 0 | 1 (4) | 1 (33.3) | 41 (6.99) | 0.243 |
| Molecular type (hans nomogram) | |||||||
| GC | 2 (33.33) | 3 (60) | 6 (50) | 10 (40) | | 274 (46.76) | 0.173 |
| Non-GC | 3 (50) | 2 (40) | 6 (50) | 9 (36) | 3 (100) | 93 (15.87) | |
| Non-classifiable | 1 (16.67) | | | 6 (24) | | 219 (37.37) | |
| Bulky disease | 0 | 0 | 0 | 0 | 0 | 338 (57.67) | 0.6 |
| Clinical stage (lugano) | |||||||
| I | 4 (66.67) | | 7 (58.33) | 1 (4) | 1 (33) | 23 (3.92) | <0.0001 |
| II | 2 (33.33) | | 3 (25) | 18 (72) | 2 (66) | 103 (17.58) | |
| III | | | | 6 (24) | | 136 (23.21 | |
| IV | | 5* (100) | 2* (16.67) | | | 324 (55.29) | |
| IPI | |||||||
| Low | 5 (83.33) | 1 (20) | 6 (50) | 4 (16) | 0 | 123 (20.99) | 0.019 |
| Low-intermediate | 1 (16.67) | 1 (20) | 5 (41.67) | 6 (24) | 1 (33) | 157 (26.79) | |
| High-intermediate | | 3 (60) | | 8 (32) | 2 (66) | 152 (25.94) | |
| High | | | 1 (8.33) | 7 (28) | | 154 (26.28) | |
| Laboratory results (mean ± SD) | |||||||
| Glucose | 108.83 ± 17.60 | 96.80 ± 9.60 | 116.00 ± 36.94 | 103.6 ± 18.5 | 102 ± 8.8 | 110.10 ± 37.90 | 0.861 |
| Creatinine | 0.69 ± 0.38 | 0.72 ± 0.20 | 0.75 ± 0.25 | 1.9 ± 1.0 | 1.03 ± 0.35 | 1.09 ± 0.40 | 0.013 |
| Albumin | 3.86 ± 0.36 | 3.70 ± 0.46 | 4.00 ± 0.32 | 3.1 ± 0.5 | 3.6 ± 0.47 | 4.10 ± 0.10 | 1.00 |
| Leukocytes | 6.75 ± 1.46 | 7.24 ± 3.75 | 7.76 ± 1.44 | 5.1 ± 2.8 | 7.54 ± 1.4 | 7.62 ± 2.40 | 0.765 |
| Hemoglobin | 13.86 ± 1.96 | 14.06 ± 1.10 | 14.10 ± 1.99 | 11.3 ± 1.9 | 13.85 ± 0.83 | 13.10 ± 2.40 | 0.003 |
| Platelets | 236.93 ± 34.60 | 358.00 ± 134.94 | 267.83 ± 101.76 | 460.5 ± 136 | 232.6 ± 0.65 | 330.00 ± 157 | 0.0001 |
| Lymphocytes | 2.06 ± 0.70 | 3.18 ± 2.46 | 1.86 ± 0.75 | 1.74 ± 0.73 | 1.840 ± 1.045 | 1.59 ± 1.10 | 0.134 |
*Because of bone marrow involvement. BMI: body mass index, ECOG: Eastern Cooperative Oncology Group, LDH: lactic dehydrogenase, HIV: human immunodeficiency virus, HBV/HCV: hepatitis B virus/hepatitis C virus-positive, GC: germinal center origin, NCG: non-germinal center origin, IPI: international prognostic index, SD: standard deviation.
Of the 6 cases in cohort A, two were asymptomatic, most had minimal symptoms without interfering with their daily activities, and most were classified as clinical Stage I or II. In accordance with Hans nomogram, the most frequent molecular type of DLBCL was the non- GC. In accordance with the international prognostic index (IPI) score, five patients (83.3%) were within the good prognosis group. Two cases of cohort B had an axial localization, two were appendicular, and only one patient had an axial plus appendicular localization. Pain, paraparesis, and bone fracture were the main symptoms. None of the patients had any nodal or other extranodal involvement. In contrast with the other subgroups, the GC molecular type was the most frequent. Most patients were within the high-intermediate group (IPI score) and most had a poor performance status.
In 5 of 12 patients from cohort C, the tumor was located within the oral cavity and in most cases within the nasal cavity and paranasal sinuses. Most of them were in clinical Stage I. Cohort D included 25 patients with primary gastrointestinal DLBCL, and the stomach was the most frequent site (n = 14), followed by colon (n = 6), small intestine (n = 3), or gastric and small intestine (n = 2). Germinal-center type was the most frequent (n = 10, 40%) followed by non- GC (n = 9, 36%) and 6 cases (24%) were unclassifiable. Mean albumin levels were lower in this group (3.1 + 0.5) but were not statistically different compared with the other cohorts. Cohort E included three patients; all were unilateral, non-germinal-center type, had B symptoms and elevated LDH levels.
Most patients in cohort F presented with B symptoms, elevated LDH levels, advanced disease and lower mean absolute lymphocyte counts.
Treatment, Response, and Survival
Patients were treated with either chemotherapy only (n = 361, 56.6%) or chemotherapy plus radiotherapy (n = 275, 43.1%), and one patient (0.15%) was treated with surgery and chemotherapy. Table 2 details the treatment modality, scheme and response in all cohorts. A higher complete response rate was documented in cohorts E and C (100% and 91%, respectively), compared with other sites of extranodal disease (cohort A: 83.3%, cohort B: 80%, or cohort D: 73.3%). It is important to emphasize that none of the patients within cohort A had bulky disease; therefore, they were treated only with chemotherapy.
Table 2 Treatment and responses according to nodal/extranodal group
| Variable | Extranodal groups | Nodal F | p-value | ||||
|---|---|---|---|---|---|---|---|
| A Breast | B Bone | C Craniofacial | D Gastrointestinal | E Testicular | |||
| Treatment n (%) | |||||||
| Chemotherapy | 6 (100) | 1 (20) | 5 (41.67) | 15 | - | 332 (56.65) | <0.0001 |
| Chemotherapy and radiotherapy | - | 4 (80) | 7 (58.33) | 9 | 1 (33) | 254 (43.35) | |
| Surgery and chemotherapy | 1 | 2 (66) | |||||
| Chemotherapy scheme n (%) | |||||||
| R CHOP | 6 (100) | 3 (60) | 10 (83.33) | 22 (88) | 3 (100) | 513 (87.54) | <0.0001 |
| R COP | - | 1 (20) | 2 (16.67) | 2 (8) | - | 36 (6.14) | |
| CHOP | - | 1 (20) | - | 1 (4) | - | 31 (5.29) | |
| DA EPOCH R | - | - | - | - | - | 6 (1.03) | |
| Response n (%) | |||||||
| CR | 5 (83.33) | 4 (80) | 11 (91.67) | 20 (80) | 3 (100) | 430 (73.38) | 0.958 |
| PR | 1 (16.67) | - | 1 (8.33) | 1 (4) | - | 41 (6.99) | |
| SD | - | - | - | 1 (4) | - | 5 (0.86) | |
| PD | - | 1 (20) | - | 3 (12) | - | 110 (18.77) | |
| Relapse | 2 (33.33) | 0 | 0 | 6 (24) | 1 (33) | 44 (7.50) | 0.078 |
| Median follow-up (months) | 26 | 21 | 25 | 32 | 16 | 27 | 0.658 |
| DFS 5-y | 42 | 100 | 100 | 76 | 53 | 60 | 0.144 |
| OS 5-y | 100 | 80 | 58 | 62 | 0.268 | ||
R CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone; R COP: rituximab, cyclophosphamide, vincristine and prednisone; CHOP: cyclophosphamide, doxorubicin, vincristine and prednisone; DA EPOCH R: dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, rituximab, CR: complete response, PR: partial response, SD: stable disease, PD: progressive disease, DFS: disease-free survival, OS: overall survival, 5y: 5-year.
The follow-up period was similar between the cohorts. Median PFS was 37 and 73 months for patients within cohorts A and F, respectively. In cohorts B, C, D, and E, the median PFS was not reached until after 5 years of follow-up (Fig. 1). During this time, the higher relapse rate was in cohort F, with 207 cases achieving a relapse rate of 35.5%. One patient (33%) within cohort E relapsed, two cases in cohort A relapsed (33.3%) at the CNS, and this relapse rate was higher and significant (p = 0.001) compared with patients from cohort F, where only 3 (0.51%) relapsed at this site. Similarly, six patients (24%) in cohort D relapsed at retroperitoneum, and no relapses were documented during follow-up in cohorts B or C. The PFS and OS are shown in Figures 1 and 2, respectively. As detailed in table 2, OS was higher in cohort A, followed by cohort B (Fig. 2). During follow-up, three non-lymphoma-related deaths were documented, as follows: two patients (cohorts B and C, one each) died because of cardiovascular related incidents, and one had an accidental death (cohort C).

Figure 1 Progression-free survival, showing that patients with primary centrofacial, and primary bone diffuse large B-cell lymphoma (DLBCL) had better survival, in comparison with the other groups of DLBCL.
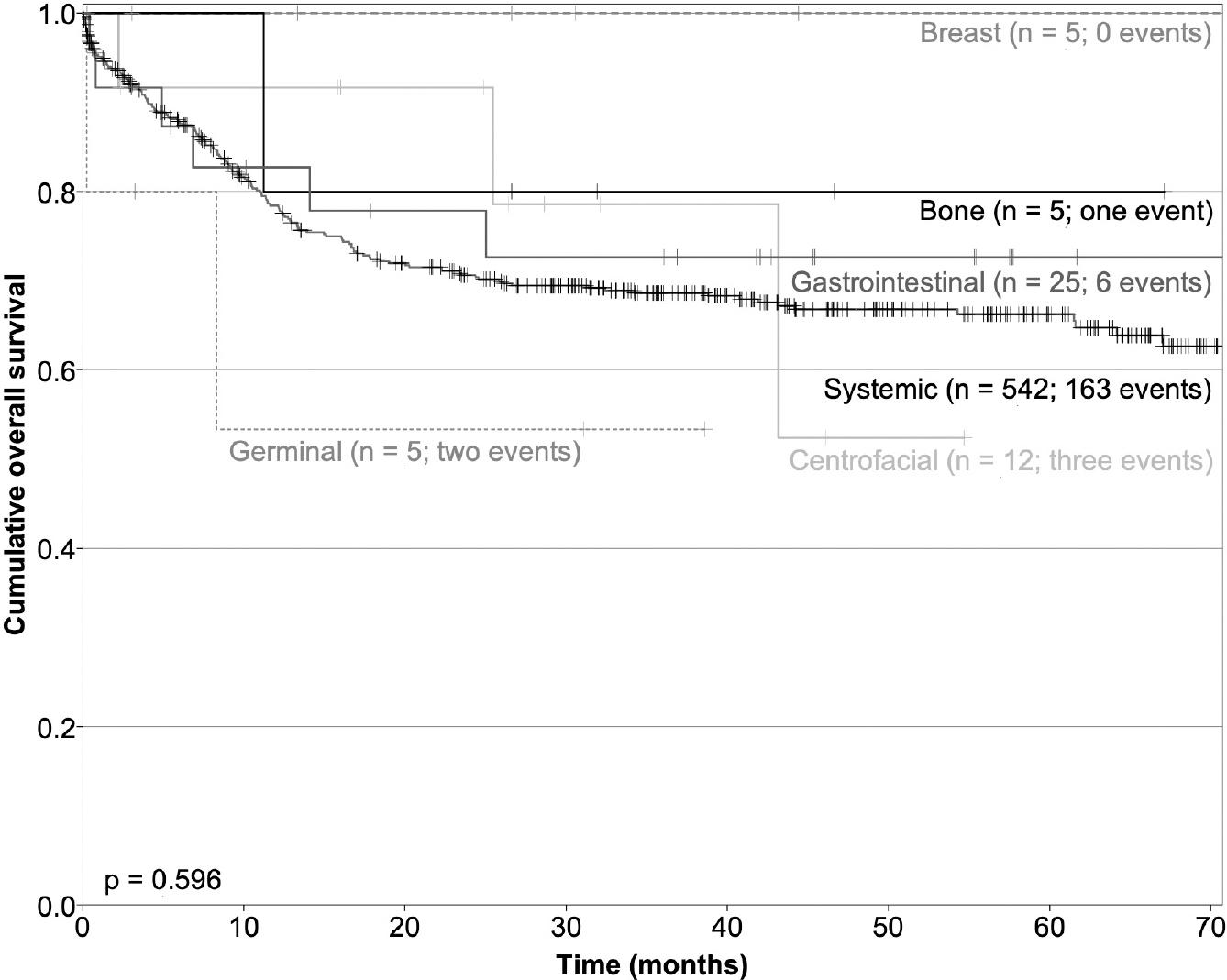
Figure 2 Overall survival was higher for patients with primary breast, primary bone-diffuse large B-cell lymphoma (DLBCL), and gastrointestinal DLBCL.
By multivariate analysis employing the Cox model, from all the clinical, biochemical, and histopathological molecular classification parameters included, none were independently associated with PFS nor OS. The achievement of a complete response was the single factor associated with PFS and OS.
DISCUSSION
PE-DLBCL is a rare and diverse pathological entity. Several efforts have been made to evaluate the clinical characteristics and management approaches of these varied presentations and they are associated with excellent disease control by employing modern therapies5,8,11,18-22.
Regarding primary breast DLBCL, the relative frequency of diagnosis was higher (0.9%) than reported by other authors (0.04-0.5%)6,18. Clinically, our findings were consistent with those reported on literature, where the median age was 53.3 years, according to the range of 40-69 years reported in most studies6,18-20,23. In contrast, with N-DLBCL, data from eight US academic institutions18, concluded that the molecular classification of DLBCL into a GC and non-GC types had no impact on survival, and other authors have confirmed these results24. In this cohort study, the most frequent subtype was non-GC, and a low-frequency of relapses occurred regardless of the molecular type (one in GC type and one in non-GC type). Different authors have reported a relapse rate ranging from 12% to 44% in the ipsilateral or contralateral breast23. Ryan reported that ipsilateral relapse occurred within 2.6 years from the beginning of treatment, whereas contralateral breast relapse occurred within up to 13.3 years. In the present study, one patient (16.6%) had bilateral involvement at diagnosis, a higher frequency than recently reported by Lalani et al.19. None of our patients had either ipsilateral or contralateral relapse after a 5-year follow-up; however, two patients relapsed at CNS (33.3%), at 8 and 36 months. This relapse rate is slightly higher than that reported on literature, of 8-29%6,18,23. The international extranodal lymphoma study group23 defined the following prognostic factors influencing OS: IPI, anthracycline-containing regimen, and radiotherapy. A negative association has also been reported between patients undergoing radical mastectomy and OS23,25,26. In this cohort study, most patients (80%) were within the low-risk group of IPI scores; all were treated with anthracyclines, and none were submitted to mastectomy. Each of these factors could have had a positive impact on OS, as shown in figure 2. In fact, primary breast DLBCL remains a rare disease, and no clear consensus concerning therapy has emerged to date. However, the most recent reports agree with the routine use of rituximab-based chemotherapy added to radiotherapy and CNS prophylaxis18,20.
Primary bone DLBCL is also a rare, primary extranodal lymphoma that occurs with a median age of onset ranging from 40-years old and is predominant in males8,11,27, as was the case in this study. The most common initial symptoms described are local pain followed by nerve compression and local mass8, as in our study: about 80% of patients had pain, 40% referred paraparesis, and 20% had a bone fracture. A study from Japan27 reported that the pelvis was the primary site involved (54%) rather than the extremities; however, Matikas et al.28 and Hayase et al.21 described the spine as the most frequent primary involved site. In our study, 40% of patients had an axial localization, 40% were appendicular, and 20% had multifocal (axial and appendicular) bone involvement. Although elevated levels of LDH have been reported in 45-70% of patients10,29, we documented normal levels of LDH in 80% of our cases. In agreement with Liu et al.30 and Hattori et al.27, most of our patients (80%) did not have B symptoms and 60% were classified within high-intermediate or high-risk groups, according to IPI score. This finding was also similar to 61% reported by Hattori et al.27. In addition, it has been reported that the presence of bone fractures is a negative prognostic factor for OS8. We had only one patient with a bone fracture, and this patient responded well to chemotherapy plus radiotherapy and remained disease-free for 3 years of follow-up. Regarding treatment, a large study from Taiwan analyzing 11 years of experience30, demonstrated that the administration of ≥ 6 cycles of chemotherapy had a positive impact on OS. The addition of radiotherapy has also been associated with an improvement in DFS, from 63% to 88%11, as well as an increase from 49% to 57.4% in OS8. All of our patients received rituximab-based chemotherapy combined with radiotherapy, and the DFS and OS to 5 years in our study were 100% and 80%, respectively.
With regard to primary craniofacial DLBCL, the differences in biological characteristics and the prognosis of lymphomas of nodal and extranodal origin of the head and neck have demonstrated a better outcome in patients with the primary extranodal disease31. In the same direction, other authors32,33 have reported that most patients with primary craniofacial DLBCL are diagnosed when they are older than 60 years, with an adequate ECOG status (0-1 groups), and low- or low-intermediate-risk groups, according to IPI score; these findings were consistent with our study, where the mean age was 67.9 years old, 75% of cases were in ECOG 0-1, and 91% were considered as low-intermediate or low-risk groups in IPI score. We found that sinonasal cavities were affected in 58.3% of patients, followed by the oral cavity in 41.7%. A study including only patients with sinonasal cavity involvement described an association with HIV or EBV infection in 32% of cases, and a clear predominance for the maxillary sinus (50%), followed by the ethmoid sinus (23%), nasal cavities (18%), and sphenoid sinus (9%). In our study, all patients were HIV negative. EBV status was not evaluated in this series. Different studies report no consensus regarding treatment. Lombard et al.22, in a study including 22 patients, treated three with localized involvement with radiotherapy, 16 patients with CHOP chemotherapy only, and the rest with combined chemotherapy plus radiotherapy. However, eight patients with an incomplete response required additional treatment, including salvage chemotherapy and bone marrow autograft (4 cases), and seven cases required complementary craniofacial radiotherapy. The OS rate was 73% at 36 months. It is important to note that patients treated with chemotherapy did not receive rituximab, which is now a standard drug in the treatment of this entity. The role of radiotherapy, as well as CNS prophylaxis in these patients, has been retrospectively analyzed by Murawski et al.34 in 11 consecutive trials of the German High-Grade NHL study group, to identify factors that affect the outcome, and they concluded that the addition of rituximab abolishes the increased risk of CNS relapse. Therefore, intrathecal prophylaxis is not recommended. In our study, 66.6% were treated with a combined modality (rituximab-based chemotherapy plus radiotherapy) and one-third with rituximab-based chemotherapy only. No relapse was documented, and our OS was 78%-5 years, which is similar to that reported in literature22,34.
With respect to primary gastrointestinal lymphoma, the GI tract is the most common site of extranodal NHL. A prospective study from South India recently published35 found that gastrointestinal lymphoma constitutes about 10-15% of all NHL. In this series, we documented only 25 cases, and this low frequency may be explained by the fact that we did not include other histologies, such as all low-grade lymphomas. As has been described35,36, our patients had a mean age of 55 years, but in contrast with the same series35,36, we documented a predominance of females; the reason for this predominance is unknown. Pain and gastrointestinal hemorrhage were the most frequent symptoms, followed by decreased appetite and weight loss, as has been reported35-37. In the same direction, the stomach was the most common site, followed by colon and small intestine. Although other series3-35 have reported DLBCL involving the esophagus, we did not find any case affected at this site.
Primary testicular lymphoma has been described as a rare, clinically aggressive form of extranodal lymphoma38, with a higher frequency in the seventh decade of life, as was in our three patients. The typical presentation with a firm mass was documented in our patients. Although HIV infection has been considered a risk factor39, all our patients were HIV-negative. The predominance of non-GC type was consistent in this cohort. The presence of B symptoms and elevated LDH levels has been defined as negative prognostic factors both were present in our 3 cases, but only relapsed. As has been described, all received chemotherapy including rituximab and one received additional radiotherapy.
These PE-DLBCLs constitute rare, primary sites of lymphoproliferative disorders in most cases, with localized disease and good prognosis. A relative lower frequency of extranodal primary DLBCL was found in this series, and it may be secondary to a referral bias since this is a national cancer center. However, they require combined chemoimmunotherapy with radiotherapy in most cases to improve the local and systemic disease.











 text new page (beta)
text new page (beta)


