INTRODUCTION
Although several pharmacological strategies have been used for the treatment of accumulation of lipids particularly in the liver as occurs in non-alcoholic fatty liver disease (NAFLD), there are now various dietary treatments that can contribute to attenuate this process1. To this end, nutrigenomics, which studies how nutrients can regulate the expression of genes involved in metabolic pathways, allows investigating whether specific nutrients may be beneficial in the prevention of certain metabolic diseases, including the excessive accumulation of lipids in several organs or tissues, to prevent lipotoxicity2. Nutrients can influence the activity of certain transcription factors, which control the expression of genes involved in specific metabolic pathways, such as lipogenesis, fatty acid oxidation, and lipid mobilization, among others3. In addition, nutrients can also modulate the expression of genes indirectly through the regulation of the secretion of hormones that can activate transcription factors by specific signaling pathways4. Both direct and indirect mechanisms converge in the transcriptional regulation of specific genes, which can modify the accumulation of lipids in the tissues. This effect can be enhanced since nutrients can also modulate the gene expression pathways at the translation or at the post-translational level5. As a consequence, there is a reduction of lipotoxicity in different organs. Therefore, the aim of the present review is to highlight the mechanisms associated with the development of lipotoxicity, and how with the advances in nutrigenomics, it is now possible to understand the molecular mechanisms by which some nutrients can attenuate the ectopic accumulation of triglycerides in non-adipose tissues, leading to the elaboration of new dietary strategies to prevent or attenuate tissue lipotoxicity. In particular, several studies using soy protein and some of its isoflavones have demonstrated that this concept can be successfully used in preclinical and clinical research6-9.
LIPOTOXICITY
Adipose tissue expandability and metabolic health
Adipose tissue plays a key role in whole-body energy homeostasis by storing excess dietary energy as triglycerides during feeding, and releasing free fatty acids to provide energy substrates during fasting, stress, or physical activity10. In addition, the mobilization of fatty acids is accompanied by an increase in the secretion of leptin by the adipose tissue. The purpose of the increase in the amount of leptin released by adipose tissue is to stimulate the oxidation of fatty acids that have accumulated in non-adipose tissues11,12. Adipose tissue mass is dynamically regulated by energy intake and expenditure13. During periods of increased energy intake, adipose tissue expands by both hypertrophy and hyperplasia to provide sufficient adipocytes for the efficient storage of triglycerides14. The main mediator of adipose tissue metabolism is the peroxisome proliferator-activated receptor (PPAR) γ, a ligand-activated transcription factor recognized as the master regulator of adipose tissue function15,16. PPARγ controls the transcriptional program of adipocyte differentiation, lipid synthesis, triglycerides esterification, lipid droplet formation, and adipokine secretion17.
Observations in animal models with altered adipose tissue function have revealed the pivotal role of adipose tissue in metabolic homeostasis18. Transgenic mice unable to recruit sufficient adipose tissue (lipodystrophic mice) develop a renal injury, hepatic steatosis, and whole-body insulin resistance19. When lipodystrophic mice are fed a high-fat diet, they do not become obese compared to wild-type mice. However, they develop type 2 diabetes many weeks earlier than their obese wild-type littermates20. Conversely, mice with increased capacity for adipose tissue recruitment become massively obese when fed a high-fat diet but are resistant to develop metabolic syndrome21. The divergent metabolic response of these mice to a high-fat diet relies on the function of their adipose tissue. Adipose tissue is known to have a limited capacity to accumulate triglycerides and this will depend on its capacity of expansion22. However, the expandability of adipose tissue is different for every individual. Thus, subjects with high capacity to expand their adipose tissue will manage better the chronic consumption of food energy than those with a limited capacity for expansion. These latter subjects quickly showed abnormalities of metabolic syndrome such as hyperinsulinemia and hyperlipidemia. When adipose tissue expansion reaches its maximum capacity, adipocytes become dysfunctional, and various metabolic consequences appear in these subjects23,24. The impaired ability of the adipose tissue to expand during chronic overnutrition is mediated by reduced activity of PPARγ. The mechanisms leading to PPARγ impairment during obesity are in part mediated by local pro-inflammatory signaling, leading to nuclear factor κB, and Jun-N-terminal kinase activation and translocation to the nucleus. As a consequence, there is a reduction in PPARγ activity, impairing adipose tissue function25.
Lipotoxicity as a common mediator in organ damage
The unrestrained release of fatty acids from adipose tissue during obesity increases their uptake in non-adipose tissues, leading to cellular damage and organ dysfunction, and a process named lipotoxicity23. Lipotoxicity occurs when an inordinate amount of fatty acids accumulates in cells in a magnitude that surpasses the oxidative capacity of the cell to utilize the excess of fatty acids. Fatty acids are then esterified leading to the accumulation of triglycerides, as well as the formation of other lipids such as ceramides and diacylglycerides. The accumulation of these lipid species causes serious metabolic damage in various organs, mainly the liver, pancreas, and heart26.
Recent evidence has demonstrated that endoplasmic reticulum (ER) stress is also involved in lipotoxic cell dysfunction and death27. The ER is the organelle responsible for the synthesis, maturation and trafficking of secretory, and membrane-associated proteins. During acute ER perturbations, activation of the unfolded protein response (UPR) mitigates ER stress by reducing protein synthesis, facilitating protein degradation, and increasing production of protein folding chaperones. However, when ER stress is excessive, prolonged, or insufficiently neutralized, the UPR activates inflammatory and apoptotic pathways28. Several basic and clinical studies have observed that during obesity, ER stress occurs as a result of the accumulation of triglycerides in non-adipose tissues, leading to lipotoxicity in the liver, skeletal muscle, heart, and pancreas, among other organs (Fig. 1).
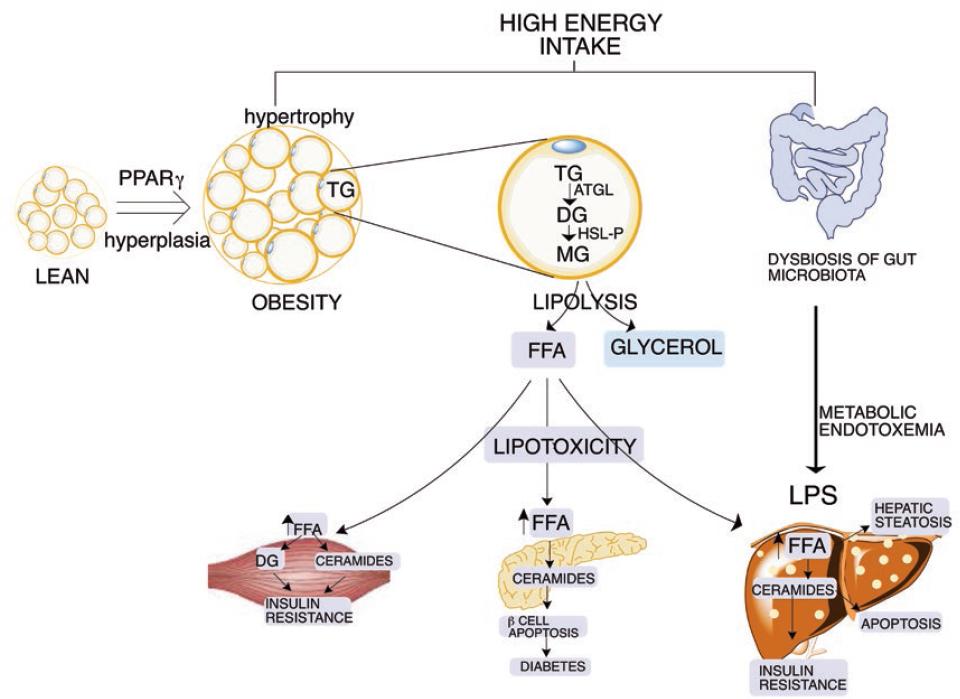
Figure 1 Development of lipotoxicity by the chronic ingestion of a high-energy diet. The adipose tissue is capable of storing the excess of energy in the form of triglycerides. At some stage, adipocytes become dysfunctional, leading to an elevated release of free fatty acids (FFA). The excess FFA are uptake by non-adipose tissues, including the liver, the skeletal muscle, and the pancreas among others, leading to lipotoxicity in different organs. The dysbiosis of the gut microbiota can enhance these effects.
During feeding periods, the circulating glucose and lipids are uptake and stored in the liver to form glycogen and triglycerides, respectively. Some of these functions are coordinated by the transcription factors sterol regulatory element-binding protein-1 (SREBP-1)29 and carbohydrate-responsive element-binding protein30. SREBP-1 is activated by insulin and induces the expression of lipogenic genes31, increasing fatty acid synthesis from glucose32. However, in obese insulin-resistant subjects, the persistent hyperinsulinemia maintains lipogenesis 5-fold higher than in healthy subjects, even during fasting33, and leading to triglyceride accumulation34. In addition to hepatic lipogenesis, the increased flow of fatty acids from adipose tissue to the liver exacerbates the accumulation of triglycerides, resulting in the development of hepatic steatosis or NAFLD, which is highly prevalent in obese subjects35.
In addition, the metabolic fate of glucose and fatty acids in skeletal and cardiac muscle is under the control of the Randle cycle, in which glucose utilization inhibits fatty acid oxidation and vice versa36. Thus, in obesity, the sustained fatty acid release from hypertrophic adipose tissue provides a continuous supply of fatty acids to skeletal muscle or heart, impairing glucose metabolism, and leading to metabolic inflexibility37. The metabolic inflexibility in skeletal muscle also reduces insulin-stimulated glucose uptake. The elevation of circulating glucose stimulates pancreatic beta cells to increase the release of insulin resulting in hyperinsulinemia38. To restore metabolic flexibility, the skeletal muscle increases mitochondrial fatty acid oxidation through enhanced mitochondrial abundance and/or activity mediated by PPARδ and by the peroxisome proliferator-activated receptor gamma coactivator 1-alpha39,40, which is activated by the enzyme AMP-activated protein kinase (AMPK)41. Leptin signaling also stimulates fatty acid oxidation and glucose uptake in skeletal muscle by activating AMPK42. However, lipotoxicity elicits ER stress in skeletal muscle, which, in turn, induces leptin resistance in muscle cells43. As a result, the mitochondrial beta-oxidation of lipids is reduced and lipid accumulation increases even further.
During obesity, the high flow of circulating levels of fatty acids from adipose tissue is also accumulated in the pancreas generating lipotoxicity44. It has been demonstrated that the excess fatty acids stimulate the synthesis of ceramides and ER stress, causing the death of pancreatic beta cells. As a consequence, there is a reduction in the beta-cell mass leading to a decrease in insulin synthesis resulting in hyperglycemia, which in the long-term triggers the appearance of type-2 diabetes45.
Lipotoxicity and the gut microbiota: dysbiosis and metabolic endotoxemia
The gut microbiota is composed of trillions of microorganisms, and their collective genomes contain > 150 times more genes than the human genome46. Studies in animal models and humans have documented a distinct composition of the gut microbiota between lean and obese subjects47. The alteration in the taxonomic proportion of the bacterial population of the gut microbiota is a condition named dysbiosis48. A gut microbiota with dysbiosis is characterized by increasing the energy harvest from the diet, fat storage, alteration of satiety signals in the brain, impairment in cholesterol and bile acid metabolism, and induction of low-grade systemic inflammation also called metabolic endotoxemia.49
Recently, it has been demonstrated that alterations in microbial communities impact liver function. An increase in pathogenic bacteria in the intestinal lumen reduces gut barrier function, leading to an increase in intestinal permeability. The reduction in enterocyte tight junctions allows bacteria to reach the portal circulation and liver. Bacteria and bacterial-derived lipopolysaccharide bind to toll-like receptor, activating pro-inflammatory signaling in the liver, leading to the progression of NAFLD50. In addition, it is known that the accumulation of lipids in the liver is associated with dysbiosis of the gut microbiota, especially with a reduction of Faecalibacterium prausnitzii and an increase in Enterobacteria51.
NUTRIGENOMICS
There is evidence that several functional foods or dietary bioactive compounds can attenuate lipotoxicity52-58. One extensively studied food is soybean, particularly soy protein. Soybean is a legume that is widely consumed worldwide. It has been established that from 1961% to 2014, 75% and 90% of the cultivated area under legumes was allocated to soybean (Glycine max) in South America and North America, respectively. In Asia, 76% of the area cultivated under legumes was allocated to four species (i.e., soybean, beans, groundnut, and chickpea). The composition of soybean has been extensively studied, and it contains between 36% and 40% of the protein in which the most abundant proteins are glycinin and β-conglycinin. In addition, soybean contains around 20% of lipids as oil, between 30% and 35% of carbohydrates, and some important minor constituents such as phytic acid and isoflavones59,60.
Soy protein has been considered a high-quality protein according to the assessment of the protein digestibility-corrected amino acid score (PDCAAS). Soy protein, after correcting for digestibility, provides amino acids equal to or in excess of requirements and receives a PDCAAS of 1, indicating that it can meet the protein needs of infants and adults when consumed as the only source of protein at the recommended level of protein intake .
Some studies have shown that the consumption of soy protein can be used as part of the dietary strategy for the treatment of several pathological conditions, including renal disease62-65 and elevated concentration of low-density lipoproteins (LDL)-cholesterol66,67. In addition, the use of soy protein has been shown to attenuate the development of lipotoxicity in several organs, as described below.
Soy protein regulates lipid metabolism in the liver by transcriptional mechanisms decreasing lipotoxicity
In the last decade, several studies have reported how certain nutrients can modulate the expression of genes involved in the accumulation of triglycerides in the liver, as is the case of soy protein68 (Fig. 2). It has been shown that soy protein can prevent the liver accumulation of triglycerides, despite the consumption of a high-fat diet. The mechanism by which soy protein regulates the accumulation of triglycerides involves the modulation of SREBP-1 activity69,70. The activation of SREBP-1 is stimulated by insulin and reduced by glucagon; therefore, the insulin/glucagon ratio determines the SREBP-1 expression and activity71.
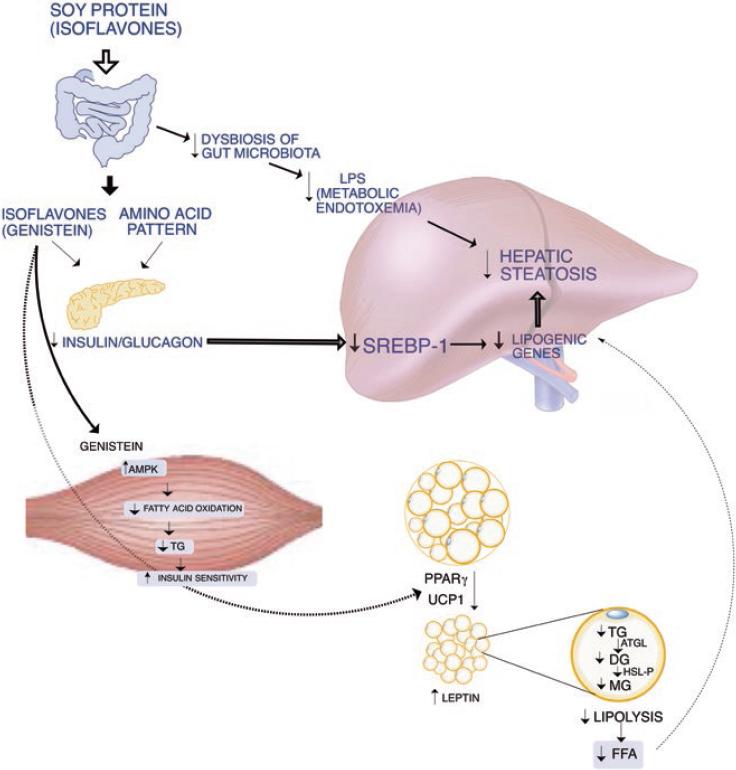
Figure 2 The consumption of soy protein and its isoflavones can ameliorate the lipotoxicity produced by the consumption of high-fat/carbohydrate diets. This effect occurs by improving the dysbiosis of the gut microbiota, by modifying pancreatic secretion of insulin and glucagon leading to a reduction in lipogenesis. In addition, adipocyte functionality improves these health benefits. Soy isoflavones can enhance fatty acid oxidation in skeletal muscle and increase white adipose tissue browning.
Some studies have shown that the increase in serum insulin produces a proportional increase in the concentration of the transcription factor SREBP-1 depending on the type of protein consumed. Rats fed a diet containing soy protein as the sole source of protein have a lower increase in serum insulin than rats fed the same percentage of a protein whose only source is casein. As a consequence, the liver of rats fed a soy protein diet expressed a low concentration of SREBP-1 mRNA and protein abundance of the mature form, as well as its target genes, such as the fatty acid synthase or the malic enzyme, among others. In contrast, the abundance of hepatic mRNA and protein abundance of the mature form of SREBP-1 were high, as well as of its target genes when rats were fed a casein diet69. This difference in the expression of SREBP-1 according to the type of protein consumed led to a lower accumulation of triglycerides and cholesterol in the liver of rats fed the soy protein diet, compared to rats fed the casein diet. These findings indicate that the type of protein consumed in the diet can modulate hepatic lipogenesis69. Interestingly, a similar outcome has been observed in Zucker fa/fa rats fed different types of proteins in the diet, despite the fact that these animals develop hyperinsulinemia. The liver of Zucker fa/fa rats fed a soy protein diet also show low levels of expression of SREBP-1 compared to rats fed a casein diet, resulting in a low accumulation of liver lipids, and mainly triglycerides72.
It has been shown that the consumption of soy protein decreases the serum concentration of insulin by reducing its secretion from pancreatic β cells. Studies in rats fed soy protein using hyperglycemic clamps as well as in vitro pancreatic islet analysis have shown that the serum amino acid pattern generated after consumption of a soy protein diet, as well as the isoflavones present in soy protein, can decrease pancreatic secretion of insulin73. On the other hand, there is also evidence that ingestion of a soy protein diet stimulates the secretion of glucagon. Therefore, the consumption of soy protein reduces the insulin/glucagon ratio causing a low SREBP-1 expression. In fact, there is a linear correlation between the insulin/glucagon ratio and the abundance of SREBP-1 mRNA (Fig. 3). In addition, recent evidence shows that genistein is capable of inhibiting voltage-dependent Kv2 potassium channels, which may be involved in part in the decrease in insulin secretion stimulated by glucose74.
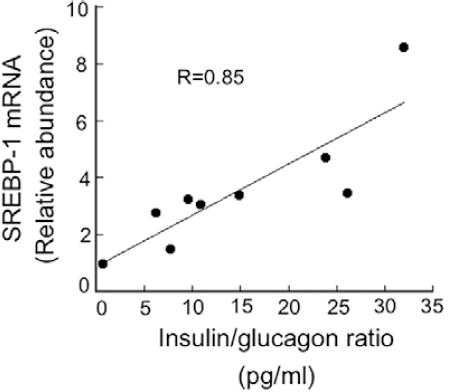
Figure 3 Association between the insulin/glucagon ratio and the concentration of sterol regulatory element-binding protein-1 (SREBP-1) mRNA in rats fed soy protein-based diets containing different percentages of fat. Male obese Zucker (fa/fa) rats had free access to the experimental diets for 2 months containing 5% soybean oil, 10% soybean oil or coconut oil, and 20% soy protein as the protein source. Serum insulin and glucagon concentration were determined by ELISA and by radioimmunoassay. Total RNA from liver samples was isolated, and mRNA levels of SREBP-1 were measured by real-time quantitative polymerase chain reaction in triplicate. HPRT mRNA was used as an invariant control.
The decrease in hepatic lipogenesis due to the consumption of soy protein compared to animals fed with casein is accompanied by an increase in the oxidative capacity of fatty acids in the liver. It has been observed that in rats fed soy protein, there is an increase in the expression of the transcription factor PPARα, which is responsible for stimulating the expression of genes involved in fatty acid oxidation75,76. As a consequence, there is an increase in the expression of its target genes such as carnitine palmitoyltransferase-1, an enzyme that facilitates the entry of fatty acids into the mitochondria to be catabolized through β-oxidation72. Thus, the consumption of soy protein favors a balance of a decrease in hepatic lipogenesis and an increase in the oxidation of fatty acids, leading to a low accumulation of triglycerides in the liver, and preventing lipotoxicity. In fact, it has been demonstrated that soy protein reduces hepatic steatosis in Zucker rats77.
Although few studies have been conducted in humans, there is evidence in subjects with NAFLD, that consumption of a diet containing soy protein can reduce the levels of alanine- and aspartate-aminotransferases, as well of serum fibrinogen, suggesting a beneficial effect in these patients78. However, long-term studies are needed to evaluate the impact of soy protein in subjects with NAFLD, particularly assessing hepatic lipid accumulation. Nonetheless, a recommended dietary strategy for NAFLD is a reduction in energy intake accompanied by the inclusion of omega-3 fatty acids and soy protein in the diet1.
The soy protein-associated isoflavone genistein prevents skeletal muscle lipotoxicity by increasing fatty acid oxidation
It has been demonstrated that the reduction in the accumulation of lipids in the skeletal muscle is associated with an improvement of insulin sensitivity79. This condition is mediated in part by the activation of AMPK leading to acetyl-CoA carboxylase 1 inactivation, possibly together with malonyl-CoA decarboxylase activation, resulting in a decrease of malonyl-CoA concentration that favors fatty acid oxidation. In addition, another function of AMPK in skeletal muscle is to stimulate GLUT4 translocation to the plasma membrane, which increases glucose uptake and metabolism80. Thus, the insulin-independent stimulation of glucose transport accompanied by an increase in fatty oxidation in muscle and heart, indicate that AMPK is a key target for pharmacological therapies in insulin-resistant subjects80. It has been described that some bioactive food compounds such as isoflavones and resveratrol can activate AMPK, stimulating glucose uptake and fatty acid oxidation in skeletal muscle. Indeed, consumption of genistein increases insulin sensitivity in obese mice associated with an increase in AMPK activity. This is associated with an increase in fatty acid oxidation in skeletal muscle, also demonstrated using C2C12 myotubes81 (Fig. 2).
Recent meta-analyses have shown that consumption of soy products and particularly soy protein in humans can be associated with a lower risk of type 2 diabetes82-84. However, there is heterogeneity of outcomes between many investigations, possibly associated with the population studied, the type of soy foods administered in the trial, and the presence of isoflavones. Nonetheless, most of the studies show an improvement in fasting glucose or insulin concentrations or amelioration of insulin sensitivity85. In addition, this evidence is in agreement with a meta-analysis study showing that the consumption of soy isoflavones can reduce fasting glucose and fasting insulin in postmenopausal women86. Further studies are needed to confirm the effect of isoflavones on glucose metabolism in humans.
Soy protein prevents adipose tissue dysfunction
Dysfunction of the adipose tissue is evident when adipocytes have a low capacity to store the excess of energy as triglycerides22. Previous studies show that body weight gain is reduced when rats are fed a high-fat diet containing soy protein, compared to those fed a high-fat diet containing casein as a protein source. The difference in body weight between groups fed with these diets is due to a lower amount of body fat in animals fed a soy protein diet71. Further studies have even shown that consumption of soy protein stimulates the number of adipocytes of smaller size, containing small lipid droplets, indicative of functional adipocytes. Thus, the concentration of circulating leptin, an adipokine secreted by the adipocytes, is lower in rats fed soy protein than in those fed casein. This process is magnified because the consumption of the type of dietary protein alters the secretion and sensitivity of the organism to leptin. This adipokine has different functions, including the hypothalamic control of food intake, in which soy protein increases the expression of the leptin receptor in the hypothalamus71. Furthermore, leptin stimulates in peripheral tissues such as liver and skeletal muscle, among others, fatty acid oxidation through the activation of AMPK87 and the transcription factor PPARα88. Studies in rats fed high-fat casein diets reveal the development of leptin resistance leading to hyperleptinemia, while rats fed soy protein show normoleptinemia indicative of normal sensitivity to the action of leptin71.
This evidence suggests that soy protein can increase the functionality of adipose tissue. Evaluation by microarray analysis in rats revealed that the consumption of soy protein modified the expression of 90 genes in adipose tissue compared with those fed a casein diet. These included genes coding for adipokines, members of the renin-angiotensin system, inflammation, and cell signaling. In addition, genes of lipid and carbohydrate metabolism were also modified, suggesting that soy protein is involved in an improvement of adipose tissue function89-91. Interestingly, one gene that is upregulated by the consumption of soy protein is PPARγ.
The reduction in the expression of PPARγ in adipose tissue during obesity leads to a decrease in the ability of adipocytes to differentiate, esterify TG, and to release adipokines22,92. It is known that several PPARγ agonists can stimulate adipose tissue functionality, increasing its capacity of expansion leading to an improvement of glucose and lipid metabolism that favors the reduction of lipotoxicity in non-adipose tissues93. Thus, the increase of PPARγ expression in adipose tissue by soy protein is associated with an enhancement of adipose tissue functionality.
Recent evidence suggests that another mechanism that can be associated with an increase in adipose tissue functionality is the effect of soy protein to increase energy expenditure. This effect is mediated by the capacity of one of the soy protein isoflavones, genistein, to stimulate browning of white adipose tissue94. Experimental evidence in cells and animal models have demonstrated that genistein can stimulate in adipose tissue the expression of the uncoupling protein 1, a protein involved in thermogenesis, as well as some of the browning markers94. Therefore, the increase of this process can stimulate the mitochondrial oxidation of fatty acids in the adipose tissue, which, in turn, can improve the functionality of the adipose tissue.
Genistein modifies gut microbiota preventing metabolic endotoxemia
Recent evidence has demonstrated that genistein can selectively modify the gut microbiota in mice fed a high-fat diet; an effect associated with a reduction in the insulin sensitivity compared to control mice fed a high-fat diet without genistein95. Interestingly, the modification in the gut microbiota was accompanied by a remarkable reduction of the circulating concentrations of LPS, preventing metabolic endotoxemia95, a condition that has been associated with the development of NAFLD96. However, more studies are needed to determine whether the modification of the gut microbiota is directly associated with a reduction in hepatic lipotoxicity, despite the consumption of a high-fat diet.
CONCLUSIONS
Overall, this review summarizes the effects of soy protein-based on nutrigenomics studies that may contribute to develop new non-pharmacological strategies for patients with metabolic diseases associated with obesity, including lipotoxicity. Thus, soy protein can prevent or attenuate non-adipose tissue lipotoxicity directly by increasing fatty acid oxidation and indirectly by enhancing adipose tissue functionality. These effects are mediated by the regulation of key transcription factors involved in lipogenesis, fatty acid oxidation, and adipocyte differentiation. Nonetheless, further studies in humans are needed to determine if all subjects have the same response to these effects of soy protein. For instance, it has been demonstrated that some individuals are more susceptible to reduce total and LDL-cholesterol with a dietary portfolio of soy protein and soluble fiber depending on the presence of some gene variants97. Interestingly, the consumption of this dietary portfolio containing soy protein by hyperlipidemic subjects has a significant effect on increasing HDL-cholesterol in subjects with genetic predisposition to have a low circulating concentration of HDL-cholesterol, a condition highly prevalent in the Hispanic population98. Notably, a recent meta-analysis conducted on the health benefits of soy protein and its isoflavones in humans has demonstrated that their consumption is safe and has no adverse effects8. Nonetheless, further studies in the field of nutrigenomics and nutrigenetics are required to provide personalized nutritional advice in which soy protein may be a beneficial food component in patients with a risk of obesity-related lipotoxicity.











 text new page (beta)
text new page (beta)


