INTRODUCTION
Irisin is a protein containing 112 amino acids, derived from fibronectin type III domain-containing protein 5 (FNDC5). It was first isolated from muscle tissue in 2012 by Boström et al., confirming the finding in 2015 by mass spectrometry1. Its name derives from Iris, the daughter of Thamus and Electra, and symbolizes the rainbow and good news from the gods to humans.
Exercise induces peroxisome proliferator-activated receptor gamma and peroxisome proliferator-activated receptor γ coactivator 1 α (PGC-1α) gene expression2. Irisin seems to be the mechanism by which such genes are potentiated3. Energy expenditure in humans prevents metabolic diseases related with obesity, such as type 2 diabetes. However, in addition to energy expenditure, there are several other mechanisms related to benefits after exercising. First, it has been observed that exercise leads to structural and metabolic changes in musculoskeletal and mitochondrial biogenesis, the muscle fiber type and angiogenesis4. Second, our group reported the important increment of the metabolic hormone fibroblast growth factor 21 (FGF21) after exercising5; FGF21 improves insulin sensitivity and lipid metabolism, and decreases glucose levels5,6. Third, exercising induces overexpression of PGC-1α, revealing an interesting browning mechanism of white adipose tissue (WAT) induced by FGF213,7. The browning of WAT induces increment in heat production and energy expenditure8,9. Interestingly, irisin is also a mechanism to increase PGC-1α expression in WAT after exercising, like FGF21 does. The Pgc-1α gene, and the protein expression in myocytes lead to a higher FNDC5 synthesis, the precursor of irisin. The circulating irisin is cleaved from FNDC5, activating the browning of WAT, increasing PGC-1α and the uncoupling protein 1, with the subsequent heat production and energy expenditure. Irisin and FGF21 are currently identified as key proteins to start the browning process, improving insulin sensitivity and energy balance in humans10,11.
Further research is needed to understand the effect of different types and intensities of exercise on circulating irisin levels, particularly after aerobic physical activity, using diverse populations and ages. In young individuals, for example, irisin increases after 30 min of exercising and decreases after 8 weeks of stopping physical activity12. In adults, the main independent circulating factors that determine irisin increment with exercise are not clear. In fact, no effect was demonstrated in the expression of FNDC5 or secretion of irisin after an increase in circulating free fatty acids or adrenaline, as it happens with FGF215,13. The association, if any, between irisin and FGF21 after exercising is, therefore, not well understood, despite both showing an important increment after physical activity5,12. We evaluated here some independent parameters that may explain the increase in irisin after exercising, including serum levels of FGF21, glucose, insulin, and liver function markers14.
SUBJECTS AND METHODS
We developed an interventional, longitudinal, and prospective study to identify significant independent parameters related to an increment of irisin serum levels after 2 weeks of supervised exercising. The Institutional Human Biomedical Research Committee approved the study and written informed consent was obtained from all participants. This clinical research was carried out in accordance with the principles expressed in the Declaration of Helsinki. Between March 2010 and August 2011, we recruited 82 sedentary healthy young adult women, without any chronic disease or medical treatment. In addition to female gender, inclusion criteria were age between 18 and 35 years old, sedentary lifestyle (defined as no regular physical activity with the intention of improving physical fitness), and body mass index (BMI) < 30 kg/m2. We excluded participants with metabolic syndrome, diabetes, dyslipidemia, hypertension, asthma, and thyroid disease. Furthermore, we excluded women with a history of cardiovascular disease or with any contraindications for exercising, as previously recommended5. Elimination criteria were cardiovascular complications detected during the treadmill test, positive pregnancy test, failure to complete all exercising treadmill tests during the 2nd week, or physical injuries. None of these were present throughout the study. Participants were evaluated before and after 2 weeks of daily supervised exercising. We did an initial physical examination and biochemical analyzes after an overnight fast of 8-12 h. The following day, participants started the treadmill test, once daily during 2 weeks, as described below. On the following day after the last treadmill, participants underwent a physical evaluation, and blood samples were collected for analysis.
Physical Activity
Patients were instructed to continue with their regular diet, lifestyle, and daily physical activities throughout the study. At baseline, we evaluated daily physical activity with a questionnaire developed by Tremblay et al. and validated in Mexican population15. The level of physical activity (kcal/day) was measured over 24 h. Each subject answered three questionnaires distributed in 2 working days and 1 day of the weekend. These results were added and the average kcal/day was obtained. Participants answered questionnaires at the beginning and the end of the study to compare baseline and final daily physical activity, confirming that questionnaires showed the expected increment due to the study protocol. Exercising was supervised following Bruces protocol, once daily (Monday-Friday) for 2 weeks. Bruces protocol consists of 7-stages treadmill test with electrocardiographic and blood pressure monitoring during exercising. Each stage lasts 3 min, and with 7 stages it gives a total of 21 min of exercising. In Stage 1, patients walked at 1.7 mph (2.7 km) with 10% treadmill inclination for an energy expenditure estimated in 4.8 metabolic equivalents (METs). The speed and inclination are progressively and automatically increased by a computer software. From the 2nd to 7th stage, the speed increases from 2.5 mph (4.0 km/h) to 6.0 mph (9.6 km/h), and the inclination from 12% to 22%. METs consumption corresponds to 7.0-22.0, respectively. One MET is 3.5 mL oxygen/kg/min, which is the average oxygen consumption of a resting individual. To carry out the activities of daily living, for example, the necessary physical activity intensity is of at least 5 METs. We used METs to define physical activity intensity with an average of < 5.9 METs as mild, 6.0-7.9 METs as moderate, and > 8.0 METs as vigorous16. Participants were divided according to the intensity reached during treadmill tests to develop a correlation analysis between irisin concentrations and intensity of exercising. Predicted maximum heart rate was calculated as 220 (210 for women) minus the age of the subject. The satisfactory response was achieved when the heart rate reached 85% of the predicted maximum frequency. Therefore, we maintained the heart rate above 85% intensity for at least 15 min in each test, unless the participant asked to stop the protocol16. To increase adherence to the study protocol, the treadmill test was performed between 07:00 and 08:30 h or between 12:00 and 14:00 h, every day. Systolic blood pressure and diastolic BP were measured at rest and every 2 min during each test. The study lasted 2 weeks for each participant with a total of 10 days of supervised exercising. Irisin levels were measured in samples obtained before and after the first and last physical activity.
Biochemical and anthropometric Measurements
The laboratory of the department of endocrinology and metabolism of our institution performed all biochemical measurements using standardized procedures. Measurements were performed with commercially available standardized and already validated methods. All equipment was regularly calibrated using reference samples provided by the manufacturer. Glucose was measured using the glucose oxidase method (Boehringer Ingelheim, Germany). Serum total cholesterol and triglycerides were measured using an enzymatic method (Beckman); HDL and LDL cholesterol levels were measured after precipitation using phosphotungstic acid and Mg2 + (Beckman). Liver tests and gamma-glutamyl transferase were measured using an enzymatic assay (Beckman Coulter, Inc.). For FGF21, we used a serum human enzyme-linked immunosorbent assay (ELISA) kit (BioVendor Laboratory Medicine, Modrice, Czech Republic). Irisin was measured using ELISA (Cusabio, catalog # CSB-EQ027943HU) with a sensitivity of 0.78 ng/ml and range between 3.12 ng/ml and 200 ng/ml, with assay procedure between 1 and 3 h, and detection wavelength of 450 nm. The irisin results were previously validated with an intra- and inter-variability assay of < 3%. Some authors claim that measuring human irisin is actually an artifact of poor antibody specificity; hence, the irisin does not really exist17. However, the molecule was detected by mass spectrometry with the same molecular characteristics as the one previously quantified in human plasma by Lee et al. with a similar assay18. Weight (kg) and body fat (%) were measured using Tanita Body Composition UM 026 Analyzer (Tanita, Tokyo). The height (cm) was obtained using a wall stadiometer. The BMI was calculated as weight (kg) divided by height (m²). Waist circumference was measured midpoint between the lower border of the rib cage and the top of the iliac crest in centimeters. All measurements were done by a standardized nutritionist blinded for study aims.
Statistical Analyzes
We used the formula for means comparison to calculate sample size. Considering a minimum difference of 10 ng/ml of irisin after exercising14, with an alpha of 0.05 (two-tailed) and study the power of 80%, a sample size of 63 women was calculated. All participants were included in each statistical analysis. Mean and standard deviation or median and interquartile range were used according to variable distribution. Log transformation was used to normalize the distribution of FGF21 and irisin. Paired Students t-test or Wilcoxon test was used to compare measurements before and after the intervention. Pearson or Spearmans correlations were used as appropriate to evaluate linear association between dimensional variables. Those with significant association were introduced to a stepwise linear regression analysis model to assess the independent determinants of irisin increment after exercising. Interaction and collinearity were evaluated with tolerance (< 0.2) and the variance inflation factor (VIF, < 5). We considered p ≤ 0.05 as significant using SPSS 22.0 (Chicago, IL).
RESULTS
A total of 82 female participants were evaluated with a mean age of 23.8 ± 3.3 years and mean BMI of 22.0 ± 5.7 kg/m2 (Table 1). Women with mild (METs mean ± DE), moderate, and vigorous activity were analyzed to compare changes in serum irisin and FGF21 levels.
Table 1 Clinical and biochemical parameters of the sample studied before and after 2 weeks of supervised physical activity (n = 82).
| Variables | Before | After | p value |
|---|---|---|---|
| Age (years) | 23 ± 3.3 | | |
| BMI kg/m² | 22 ± 5.6 | 21.6 ± 6.1 | 0.34 |
| WC (cm) | 75.4 ± 7.7 | 75.0 ± 7.6 | 0.09 |
| Fat mass (%) | 27.7 ± 5.3 | 28.2 ± 8.2 | 0.91 |
| Free fat mass (%) | 45.2 ± 16.5 | 45.2 ± 16.7 | 0.76 |
| WHR | 0.81 ± 0.05 | 0.81 ± 0.71 | 0.62 |
| ALT (U/L) | |||
| Baseline | 15.2 ± 6.8 | 14.9 ± 7.8 | 0.04 |
| After moderate exercising | 15.2 ± 6.8 | 18 ± 5.2 | 0.04 |
| After vigorous exercising | 15.2 ± 6.8 | 22 ± 1.9 | 0.02 |
| AST (U/L) | 20.3 ± 4.6 | 19.6 ± 5.2 | 0.33 |
| GGT (U/L) | 14.2 ± 6.9 | 13.4 ± 5.5 | 0.01 |
| HDL mg/dL | 52.6 ± 12.8 | 53.9 ± 13.7 | 0.21 |
| Insulin (µUI/mL) | 8.3 ± 4.0 | 7.7 ± 4.0 | 0.13 |
| Glucose (mg/dL) | 85.9 ± 6.6 | 85.6 ± 6.3 | 0.91 |
| Irisin (ng/mL) | 158 (110-230) | 211 (124-292) | 0.02 |
| DBP (mmHg) | 66.7 ± 6.3 | 65.8 ± 6.0 | 0.18 |
| SBP (mmHg) | 103.7 ± 10.2 | 102.4 ± 8.9 | 0.15 |
| TGS (mg/dL) | 105.76 ± 60.56 | 93.19 ± 61.68 | 0.005 |
| FGF21 (ng/L) | 276.8 (142.8-568.6) | 460.8 (298.2-742.1) | 0.0001 |
PRE: pre-exercise, V2: visit 2 or after exercise, WHR: waist-hip ratio, BMI: body mass index, WC: waist circumference, ALT: alanine aminotransferase, AST: aspartate aminotransferase, GGT: gamma-glutamyl transferase, HDL: high-density lipoprotein, TGS: triglycerides, DBP: diastolic blood pressure, SBP: systolic blood pressure, FGF21: fibroblast growth factor 21. *Data are presented as mean ± SD or median (interquartile range)
Irisin and FGF21 Levels Increased After Exercising
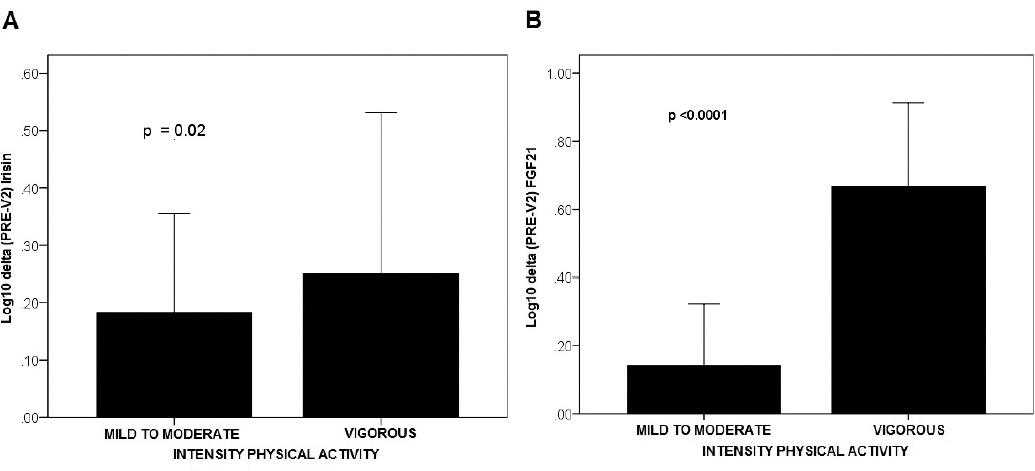
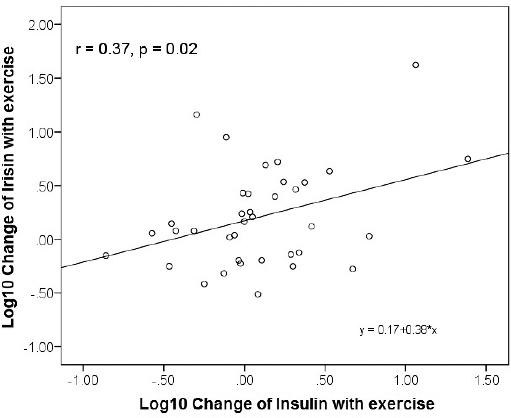
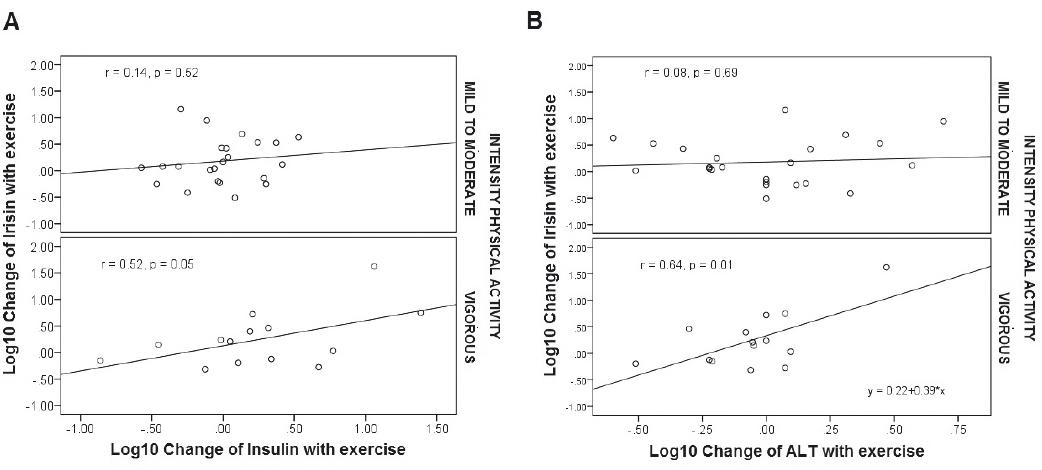
A significant increment on irisin after exercising was documented (before 158 [110-230] vs. 211 [124-292] ng/mL, p = 0.02, Glass-delta = 0.25) (Fig. 1). In addition, serum FGF21 levels were also higher after 2 weeks of exercising (before 276.8 [142.8-568.6] vs. after 460.8 [298.2-742.1] ng/L, Glass-delta = 0.77, p = 0.0001) (Fig. 1). Consistently, a significant correlation was identified between irisin and exercise intensity (r = 0.37, p = 0.02; Fig. 2), as well as physical activity intensity and FGF21 levels (r = 0.40, p < 0.001). No significant correlation, however, was identified between FGF21 and irisin. We identified a positive and progressive increment in alanine amino transferase levels (ALT), with particularly high correlation with irisin after vigorous exercising (r = 0.64, p = 0.01; Fig. 3). Irisin also positively correlated with insulin depending on the intensity of physical activity (r = 0.52, p = 0.05; Fig. 3).
Independent Determinants Related with Irisin Increment
Linear regression model adjusted for glucose and BMI was performed to evaluate the independent determinants of irisin increment after exercising. Multivariate model results showed a significant and independent association between ALT and irisin, as well as insulin and irisin (adjusted R² = 0.121, p = 0.04). When individuals were divided into groups of mild to moderate and vigorous exercise, we identified a significant and independent association with vigorous exercising (adjusted R² = 0.46, p = 0.03; Table 2).
Table 2 Linear regression model to evaluate the independent determinants of irisin increment after intensive exercising.
| Independent variable | β | Standardized β | t | p |
|---|---|---|---|---|
| Insulin | 0.350 | 0.331 | 2.092 | 0.044 |
| ALT | 1.16 | 0.050 | 1.92 | 0.008 |
| BMI | 0.38 | 0.092 | 0.819 | 0.415 |
| Glucose | 0.019 | 0.014 | 0.109 | 0.912 |
Model summary: R² = 0.46, F = 4.7, p = 0.03.ALT: alanine aminotransferase. Interaction and collinearity were confirmed with tolerance < 0.2, and VIF < 5. VIF: variance inflation factor, BMI: body mass index
DISCUSSION
This study evaluated the significant and independent parameters related to an increment on irisin after 2 weeks of supervised physical activity. We found that intensive exercising caused a significant increment in serum irisin (Fig. 1a) and FGF21 (Fig. 1b) levels; however, since no significant correlation between them was identified, it could be explained by independent mechanisms. Furthermore, after vigorous exercising, the independent determinants of such increase in irisin were ALT and insulin (Table 2).
Irisin levels are influenced by age, sex, obesity, and metabolic conditions. In addition, intensive exercising acutely and transiently increases the concentration of irisin in children and adults19. Irisin also increases immediately after different types of physical activity, declining 1 h later20. The increment of irisin has been described in healthy individuals as well as in those with metabolic syndrome. Although irisin has been widely studied after exercising, currently the main determinants of its increment are unknown, along with the reasons explaining why such response may contribute to the beneficial effects of exercising in patients with the metabolic syndrome20.
In a pilot study, irisin levels increased by 35% after only 3 min of starting physical activity21. Irisin also increased after exercise-induced muscle damage from downhill running22, but not with resistance training23. Consistently, irisin levels increased after 2 weeks of exercising in our cohort of young women. Based on this and a previous study, exercise induces the secretion of myokines, like irisin, and also FGF215,24. We confirmed that irisin levels were significantly different between exercise workloads. We also confirmed that FGF21 increased particularly after vigorous exercising, as previously reported by our group and others5,25. However, the correlation between FGF21 and irisin levels after exercising was not significant, suggesting an independent regulation for each. The Fgf21 knock-out mice model induced increased body weight, adiposity, higher concentrations of serum cholesterol, insulin, and glucose (p < 0.05)26. In addition, the mitochondrial enzyme beta-hydroxyacyl-CoA dehydrogenase and the nuclear contents of PGC-1 alpha in the liver were 30-50% lower in FGF21-deficient mice (p < 0.01), suggesting a key role of FGF21 in hepatocyte metabolism26.
Our results also involve another liver enzyme in FGF21 and irisin physiology after exercising. ALT positively correlated with both, FGF21 and irisin levels, after 2 weeks of physical activity. Therefore, we believe that the liver plays an important role in regulating FGF21 and irisin effects after physical activity. Previous reports also associated insulin as an independent regulator of circulating FGF21 levels7. Since insulin was strongly and independently associated with irisin after vigorous exercising, FGF21 and irisin action in the liver may be related to the regulation of insulin sensitivity (Figs. 2 and 3a). Therefore, after exercising, FGF21 and irisin increase to improve insulin sensitivity using independent mechanisms but perhaps with additive effects27,28. Furthermore, lower irisin and FGF21 levels have been reported in newly diagnosed type 2 diabetes in comparison with healthy subjects7,30,34. The increment of both hormones may counteract their reduction caused by insulin resistance states, improving liver metabolism, reducing hepatic gluconeogenesis, increasing PGC-1 alpha, and improving mitochondrial production of heat, with the subsequent energy waste. These beneficial effects of FGF21 and irisin occur after different stimuli, including conditions with higher noradrenaline release, cold exposure, shivering, and exercising3,9,18,29,31. Insulin plays an important role in such conditions too, and therefore, it is interesting that now we have found a significant and independent relationship between insulin and both FGF21 and irisin after physical activity.
Considering our results and those of others, the increment of FGF21 and irisin could be a good marker of improved cell metabolism and higher insulin sensitivity after exercising. Although higher levels of ALT could be explained by other reasons (including liver disease under an insulin resistance environment, e.g., liver steatosis), it is important to realize that ALT levels increased but remained under the normal reference range (Table 1), making this a rare possibility. In contrast, we found a strong correlation between ALT and irisin, particularly significant after vigorous physical activity (Fig. 3b). Our findings suggest that ALT increment is a consequence of irisins effect on liver, as a mediator of the beneficial effects of exercising on liver cell metabolism. Moreover, in patients with obesity, irisin concentrations have been found to be independently associated with liver fat content (p < 0.01)30. FGF21 has also been related with a positive association with fat liver content before cirrhosis34-36. When the distribution of intrahepatic triglyceride content (IHTG) was quantified, a significant increment in serum irisin and FGF21 was demonstrated, after decreasing the IHTG content (p < 0.01)30,34-36. Therefore, the increment of serum irisin and FGF21 levels after exercising may help to improve hepatocyte metabolism.
The lack of correlation between FGF21 and irisin warrants further research. We previously reported that FGF21 positively correlated with free fatty acids and noradrenaline after exercising5. However, a post hoc correlation between serum irisin and FFA in our new cohort was not significant (r = 0.11, p = 0.81), which suggests different regulation mechanisms of irisin increment.
One important strength of our study is that we used a supervised and standardized exercise test to evaluate the impact of aerobic activity intensity on irisin and FGF21 levels. However, some limitations of the study are that we did not study the effect of exercising in patients with overweight or obesity. Furthermore, we still do not know what is the long-term effect of increasing circulating irisin and FGF21 levels after exercising.
In summary, irisin and FGF21 concentrations are influenced by the intensity of physical activity. This effect should be taken into consideration for future clinical studies evaluating circulating irisin and FGF21 levels. Furthermore, serum ALT levels were identified as an independent determinant for circulating irisin levels. This finding suggests a positive irisin effect of exercising on liver cell metabolism.











 nueva página del texto (beta)
nueva página del texto (beta)