INTRODUCTION
Individuals who smoke cigarettes or who smoked in the past may develop a variety of respiratory alterations and diseases. Chronic-obstructive pulmonary disease (COPD) is one of the consequences of smoking, causing millions of deaths and disease in the world, and is the third most common cause of death worldwide. However, smokers may develop diseases not conforming to the classical COPD definition with airflow obstruction detected by spirometry. For example, smokers may develop chronic bronchitis, chronic cough or phlegm, or be without airflow obstruction, that is, non-obstructive chronic bronchitis. Individuals with emphysema detected on computed tomography (CT) may also lack airflow obstruction. Recently, this population of smokers without airflow obstruction has been characterized again and is the theme of this review.
Airflow obstruction is defined by spirometry as a reduced post-bronchodilator forced expiratory volume at one second (FEV1)/forced vital capacity (FVC) ratio, and low FEV1/FVC was defined by the global initiative for chronic obstructive lung disease (GOLD) criteria as < 0.70. More recently, the GOLD classification has included symptoms and exacerbations due to their importance, and also due to the lack of accessibility to spirometry testing in different regions and countries.
The prognostic and therapeutic implications in symptomatic smokers without COPD, beyond the fact that they should urgently suspend smoking as a first priority, are a controversial field that remains under discussion. This is because symptoms do not precisely and necessarily predict the development of COPD: 80% of smokers evade COPD and another percentage of subjects lose pulmonary function despite being asymptomatic. One perspective is focused on how smokers avoid COPD in terms of the mechanisms of inflammation and the immune response, and the second is centered on the observation of several cohorts, defining the proportion of the different populations that acquired COPD over a long time-period. It is not clear, which is the percentage of smokers who have a number of symptoms (cough, sputum, wheezing, and dyspnea), as well as abnormalities in other tests such as imaging, lung function, and inflammation markers1,2.
There is less information available on changes in inflammatory variables such as biomarkers and the histology/morphology in airways and parenchyma of non-obstructed smokers compared with normal non-smokers and COPD patients. Likewise, scarce information has been published on lung function testing, apart from spirometry, carbon monoxide diffusion capacity (DLCO), small airways obstruction by impulse oscillometry, pulmonary volumes, and exercise performance in this type of smokers. Furthermore, information is scant regarding the frequency of emphysema, airways remodeling, air trapping, bronchiectasis, and fibrosis in this group1,2.
All available evidence leads not only to search for interventions to preserve lung function and quality of life and avoid exacerbations, above all, through smoking cessation, but also to find possible medications that may help to reach these objectives.
INFLAMMATION AND MORPHOLOGY BEFORE THE APPEARANCE OF OBSTRUCTION
Smokers have small airways disease (SAD), goblet cell metaplasia, increase in the proportion of bronchial gland mass, smooth muscle hypertrophy, inflammation in the walls and glands of bronchioles (macrophages and neutrophils), respiratory bronchiolitis, alveolar wall thickening, and an excess of airways < 400 µ in diameter, correlating with the extent of centrilobular emphysema, compared with non-smokers3-5. For most pathologic features examined (inflammation, fibrosis, muscle scores, and goblet cells number), there is an orderly progression in severity when comparing smokers to non-smokers and COPD to smokers3-5 (Fig. 1).

Figure 1 Surgical specimen of a 78-year-old female smoker (60 packs/year) with normal spirometry (no obstruction in forced expiratory volume at one second [FEV1] = 115%p and FEV1/forced vital capacity ratio = 0.85), normal diffusion capacity (88%p), and air entrapment in plethysmography (residual volume = 178%p), whose sample shows small airway disease (airway wall thickness, epithelial metaplasia, basement membrane thickness, smooth muscle hypertrophy, and outer lawyer fibrosis) and parenchymal emphysema (destroyed alveolar attachments and enlarged airspace).
Normal (non-obstructed and asymptomatic) cigarette smokers may have an inflammatory reaction involving the entire tracheobronchial tree (central and peripheral airways, lumen, parenchyma, and pulmonary arteries) and alveolar wall. T-lymphocytes and macrophages are the predominant cells infiltrating the airway wall, whereas neutrophils, which are scarce in the airway wall, are increased in the airway lumen, while mononuclear cells, CD4+ T-Lymphocytes and clusters of macrophages are observed in the wall of peripheral airways6,7.
In healthy, non-obstructed smokers, the number of CD8+ T-lymphocytes showed a significant negative correlation with FEV1 as did the value of smooth muscle area normalized by the internal perimeter, in the same way, that occurs in COPD smokers8. For a given perimeter of the basement membrane, the luminal diameter, total wall area, smooth muscle area, destroyed alveolar attachments and airway external perimeters are similar in non-obstructed smokers and COPD smokers. Airways responsiveness and air trapping are associated with airway luminal size, total airway wall thickness and airway smooth muscle thickness in non-obstructed smokers as in those with COPD9. Sputum biomarkers relevant for airway inflammation (myeloperoxidase, neutrophil gelatinase-associated lipocalin, YKL-40, interleukin [IL]-6), in healthy smokers have intermediate levels between those from non-smokers and those with COPD10.
RESPIRATORY SYMPTOMS AND QUALITY OF LIFE IN NON-OBSTRUCTED EVER-SMOKERS
Several studies have explored the prevalence of various respiratory symptoms and the risk factors for their appearance. In the IBERPOC study in Spain, the percentage of cigarette smokers with a cough was 31.8% and with sputum 22.7%, versus 5% and 3% in non-smokers, respectively11. In another study, the risk of dyspnea (odds ratio [OR]) increased with the number of cigarettes smoked per day: 1-14 cig/day = 1.89, 15-24 cig/day = 2.98, and > 24 cig/day = 3.57; women were more prone to have dyspnea Grade 3 compared to men (11.7 vs. 10.3%)12. Former smokers also developed dyspnea, as Grade 3 was present in up to 46.7% of men and 12.8% of women12. In a study from Iran, habitual smoking was a predictor of the presence of cough (OR, 2.27), breathlessness (1.88), phlegm (2.9), and chest tightness (1.41) compared to never smokers; this corresponds to a prevalence of cough of 41.8%, phlegm 40.5%, breathlessness 18.9%, and chest tightness 10.6%13. Former smokers may also be symptomatic: cough was present in 30.2% (OR = 1.31), phlegm in 24.5% (OR = 1.45), breathlessness in 18.5% (OR = 1.69), and chest tightness in 11.9% (OR = 1.56)13.
In a group of non-obstructed ever-smokers (42.1% still smoking), the proportion of individuals with cough and phlegm was 27.4%, morning cough 20%, chronic cough 15.6%, wheezing attacks 8.3%, and dyspnea Grade > 1 (modified medical research council scale) was 14.5%14.
The quality of life in GOLD stage 0 (smokers with symptoms and normal spirometry) measured by the St George Respiratory Questionnaire (SGRQ) and the short form-36 generic scale, is significantly affected: SGRQ symptoms domain score for GOLD 0 was 27 and for non-smokers, 12.4 (the higher the score and the worse quality of life). A dyspnea score of > 1 was present in 23.5% of GOLD 0 smokers in contrast to 3.7% of never smokers; chronic bronchitis was present in 12.6% of GOLD 0 and 4.3% had severe exacerbations compared to none of never smokers15.
In a study of 2736 current or former smokers and never-smoker controls to whom the COPD assessment test (CAT) was applied, respiratory symptoms were present in 50% of current or former smokers with preserved pulmonary function, an intermediate prevalence between never smokers and GOLD mild/moderate group16. The prevalence of chronic bronchitis in asymptomatic current or former smokers with CAT < 10 was 6% and in those with CAT ≥ 10 was 33%; likewise, for wheezing was 32% and 69% in current or former smokers with CAT < 10 or ≥ 10, respectively. Symptomatic current or former smokers had more respiratory exacerbations (0.27 ± 0.67 mean and standard deviation events per year) than asymptomatic current or former smokers (0.08 ± 0.31) and never smokers (0.03 ± 0.21) (p < 0.001 for both comparisons)16,17.
Respiratory symptoms are often treated although no medication recommendation exists: 20% of symptomatic GOLD 0 or impaired smokers used a respiratory medication (inhaled long and short β agonist 17.3%, inhaled anticholinergics 6.6%, and inhaled corticosteroids 7.7%); and 5% of asymptomatic GOLD 0 used a respiratory medication (4.2%, 0.6%, and 1.8% according to the kind of inhaled medication, respectively). GOLD 0 individuals who were users of respiratory medications were more commonly African-American women, with chronic bronchitis, more exacerbations, and dyspnea15.
Among symptomatic (CAT ≥ 10) current or former smokers, 42% used bronchodilators and 23% used inhaled glucocorticoids; other medications were used less commonly and lacking a solid basis: antibiotics and antibiotic/glucocorticoid combination. These patients have more visits to medical offices and hospitals than asymptomatic (CAT < 10) current or former smokers, and current or former smokers with COPD with CAT < 1016.
PULMONARY FUNCTION AND PHYSICAL ACTIVITY IMPLICATIONS IN NON-OBSTRUCTED SMOKERS
It has been clear for a long time that ever-smokers may often have respiratory symptoms and normal spirometry, but it should be investigated whether they have other changes in lung function, since spirometry evaluates only mechanical alterations. Pulmonary compliance (static pressure-volume) and total lung capacity measured in a body plethysmograph increase rapidly in cigarette smokers18.
In a group of current and former smokers, those with CAT < 10 walked 461 m in the 6-minute walk test (6MWT) compared with 410 m among those with CAT > 10. Non-obstructed smokers (or ex-smokers) had shorter 6MWT (447 m) than never smokers (493 m)15. On the other hand, in another study, DLCO was normal (7.5 mmol/min/KPa) in non-obstructed smokers even though 42% were still smoking14. In a cohort study of non-obstructed ever-smokers (25% active smokers) with normal or low diffusion capacity (DLCO = 68%p), after 3 years of follow-up, 22% of those with low DLCO developed obstruction, compared with 3% of the normal spirometry/normal DLCO group19.
Symptomatic ever-smokers, regardless of asthma history, had slightly lower FEV1 (94.1%p) and response to bronchodilator (7.4%) than asymptomatic ever-smokers (98.5%p and 6.1, respectively), with greater limitation of activity16.
Respiratory symptoms and peripheral airway dysfunction are common in smokers with normal spirometry: 76% of the subjects had an abnormality either in the multiple breath nitrogen washout or the impulse oscillometry system (IOS)20. Chronic bronchitis symptoms were associated with conductive airway abnormalities, while wheeze was associated with an increased resistance. In another study of non-obstructed smokers, the prevalence of SAD evaluated by IOS was 26.3%, and they had lower spirometry values, poorer quality of life, and higher levels of C-reactive protein21. Abnormal IOS test has also been associated with inflammation in bronchoalveolar lavage: two-fold higher lymphocyte and neutrophil counts and higher IL-8, eotaxin, and fractalkine levels. Reactivity of distal obstruction and reactance by IOS correlated with levels of IL-8, eotaxin, fractalkine, IL-12p70, and transforming growth factor-α22.
IMAGE CHARACTERISTICS AND FINDINGS IN SMOKERS
CT of the thorax is more sensitive for detecting lung abnormalities, and current software allows to perform accurate airway and parenchymal measurements, such as airway wall thickness, and calculate the percentage of lung with emphysema (low attenuation areas, density < −95 HU, % LAA-950). GOLD 0 smokers have been shown to have thicker airways than never smokers (32.2 vs. 7.4%). Non-obstructed smokers (GOLD 0) had more emphysema (24%), airways thickening (30.7%), or any of those (42.3%) than never smokers (3%, 9%, and 10%, respectively)15. Symptomatic (CAT > 10) non-obstructed ever-smokers had more airways disease by CT not only than never smokers but also than asymptomatic ever-smokers or asymptomatic mild-moderate COPD16. In this study, no increase in emphysema was seen (< 2% emphysema) in ever-smokers with preserved spirometry regardless of the CAT score16. Emphysema and increased airways wall thickness (AWT) by high-resolution CT (HRCT) were already found as remodeling manifestations in non-obstructed smokers (Fig. 2). The median (25-75th percentile) percent of low-attenuation area was minimum, an extent of 0.71% and 0.32% in males and females, respectively. Interestingly, the extent of emphysema was greater in ex-smokers than in current smokers. For airways findings, the mean AWT was 0.488 and 0.463 mm in males and females, respectively, similar to the COPD group23.
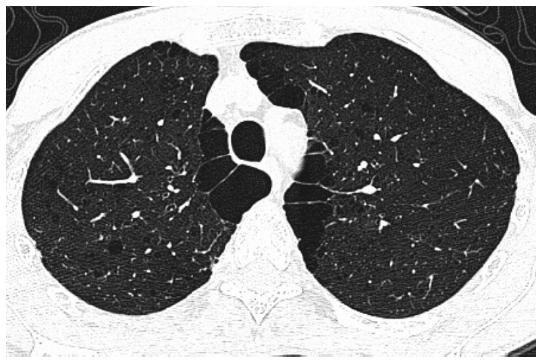
Figure 2 High-resolution computed tomography of the thorax of a 57-year-old male smoker (22 packs/year) with normal spirometry (no obstruction forced expiratory volume at one second [FEV1] = 80%p, and FEV1/forced vital capacity ratio = 0.86), normal diffusion capacity (78%p), air entrapment in the plethysmography (residual volume = 149%p), and no dyspnea or desaturation in 6-minute walking test; image demonstrates centrilobular emphysema, subpleural bullae, and subsegmental airways wall thickness.
In non-obstructed smokers (current smoking of 42.12%), the median (25th-75th percentile) percent low-attenuation areas < −950 HU (% LAA-950) were also related to minimum emphysema of 0.5, and mean AWT at an internal perimeter of 10 mm (AWT-Pi10) was increased in subjects without COPD. This AWT-Pi10 was significantly related to wheezing in subjects without COPD. OR for increased dyspnea was 1.9/10% increase in % LAA-950 and 1.11/0.1-mm increase in AWT-Pi1014. This remodeling, although small, was associated to symptoms.
In a large group of smokers, where 39% of participants were GOLD = 0, interstitial lung abnormalities (ILA) were present in 8% of HRCT scans evaluated in a group with more exposure to tobacco and current smoking, more likely to have a restrictive lung deficit (total lung capacity < 80% of the predicted value; OR = 2.3), and less likely to meet the diagnostic criteria for COPD (OR = 0.53)24. Those participants with GOLD = 0 had a high prevalence of centrilobular emphysema (44%), mixed centrilobular and subpleural emphysema (36%), and ILA (39%)24.
CONCLUSIONS
Non-obstructed smokers may have a variety of alterations in lung morphology and function and persistent inflammatory abnormalities. Respiratory symptoms and acute exacerbations in non-obstructed current or former smokers are common, with an adverse impact on quality of life. Lung function, exercise tolerance and CT scanning abnormalities such as bronchial wall thickening, or emphysema may also be present.
It is controversial whether or not this population should be treated with medications besides intensive efforts to stop smoking. Further studies should be done to elucidate follow-up and prognosis issues.











 nueva página del texto (beta)
nueva página del texto (beta)


