INTRODUCTION
Sixty years ago, the term emphysema in the United States was equivalent to chronic bronchitis in Great Britain; to avoid confusion, the former was considered an anatomopathological diagnosis, while the latter was a clinical diagnosis. This underscores the controversy that has since existed to define and diagnose the disease named chronic obstructive pulmonary disease (COPD). COPD is one of the main causes of morbidity and mortality in the world1. Today, it is the third most important non-transmissible disease, representing 5.3% of all deaths worldwide2.
INFORMATION SEARCH
We searched for manuscripts published and indexed in PubMed under the following terms: COPD or COPD and diagnosis and definition excluding asthma and overlap syndrome, considering review articles and clinical practice guidelines published during the past 10 years. We obtained 97 articles relevant for the purpose.
Definition
According to the definition proposed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) in the Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease, 2017 update, COPD is a common, preventable, and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases1.
COPD definition has been changed during the years. Today, it is accepted that COPD is not only a single clinical entity but also is considered a complex syndrome, resulting from the chronic exposure to one or more noxious agents that are known and that generate a different clinical course1. The definition of COPD has included a functional component, centered originally on the progressive and accelerated decline in lung function in individuals who smoke, and now on irreversible airflow obstruction, as clearly airflow obstruction may occur by the hastened decline of forced expiratory volume in 1 second (FEV1) or by abnormal growth and development of the lung3. At least in epidemiological studies, individuals with irreversible airflow obstruction without relevant exposures are included in the COPD category, which may give rise to confusion. In addition to functional abnormalities, individuals with COPD present varying degrees of emphysema and chronic bronchitis. This complex syndrome is characterized by inflammation not only of the lungs and airways but also systemic4, which leads to an increased risk of comorbidity, functional deterioration, as well as limitations in performing daily life activities and decrease in the health-related quality of life5.
Risk factors for COPD include a deficiency of natural antiproteases (α1-antitrypsin or antiprotease); exposure to tobacco, biomass, or industrial smoke; previous pulmonary infections; asthma; and abnormal pulmonary development caused by prenatal or early life events6. The majority of cases of COPD in the developed world are related to tobacco consumption; thus, its importance should be emphasized as a definite and preventable cause of the disease. However, about one-third of patients with COPD, or more correctly with irreversible airflow obstruction, are individuals who never smoked7.
COPD has been clinically defined by the presence of some cardinal symptoms that include dyspnea, cough, and sputum production6,8,9. GOLDs Global Strategy for the Diagnosis, Management, and Prevention of COPD, in its most recent 2017 update, mentions that the diagnosis of COPD should be considered in all individuals with dyspnea, chronic cough, or phlegm and/or exposure to any of the risk factors for the disease1. However, recent studies have demonstrated that some subjects who are smokers experience symptoms similar to those observed in patients with COPD - they even exhibit episodes resembling a COPD exacerbation - but without airflow obstruction6. In some of these patients, computed tomography (CT) of the thorax has demonstrated pulmonary emphysema; although these individuals do not fulfill the diagnostic criteria of COPD proposed by GOLD, they have a pulmonary disease associated with smoking or with another exposure (Fig. 1)10. Due to the possible progression to airflow limitation, this disorder was named pre-COPD6, which was recognized by GOLD since 2006 as Stage 0. However, this questionable term disappeared in future revisions, since not all subjects with GOLD 0 (or pre-COPD) will develop airflow obstruction11.
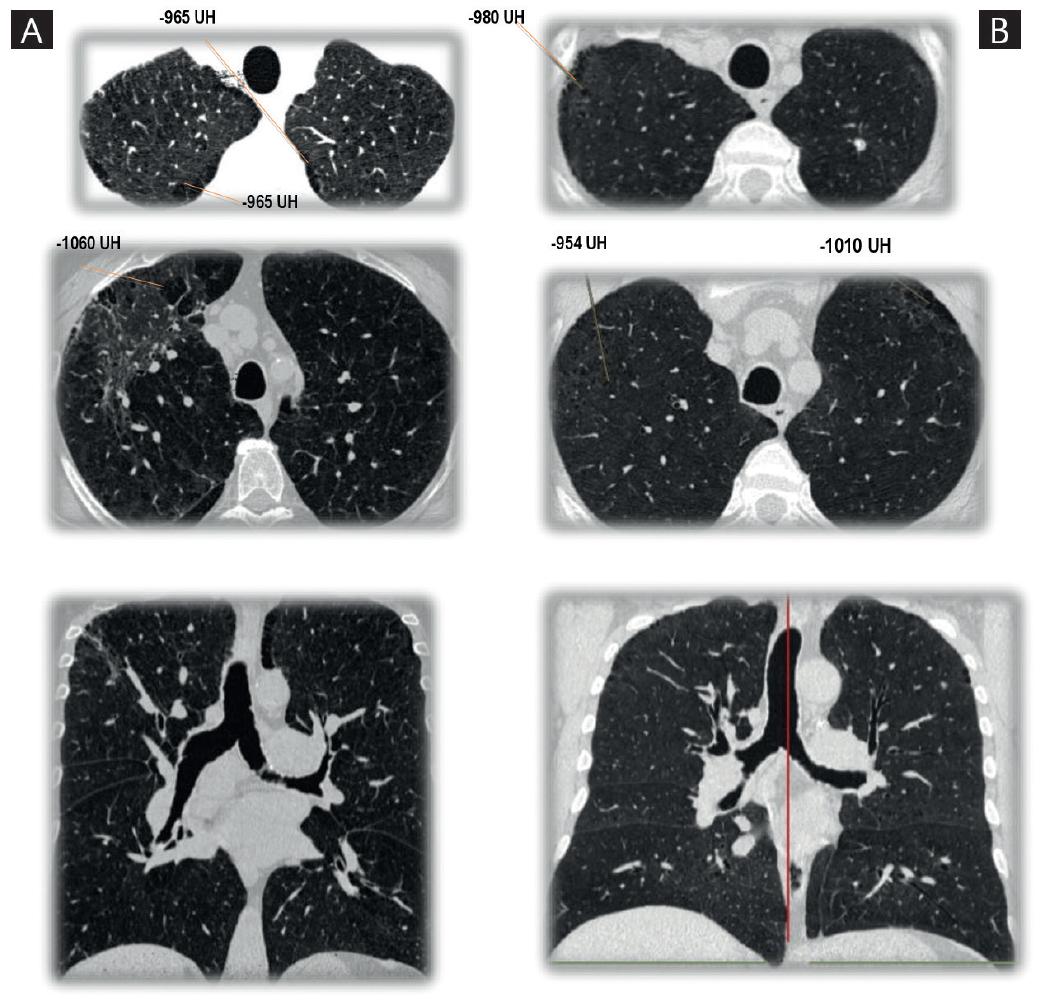
Figure 1 A: 60-year-old male with chronic obstructive pulmonary disease diagnosis. History of smoking for 35 years, 20 cigarettes a day. Postbronchodilator spirometry: forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) 58%, FEV1 68% predicted. High resolution thorax tomography shows hypodense areas in apices with -950 UH suggestive of centrilobular and paraseptal emphysema. B: 51-year-old male with chronic cough and dyspnea. History of smoking for 28 years, 16 cigarettes a day. Postbronchodilator spirometry: FEV1/FVC 74%, FEV1 92% predicted. High resolution thorax tomography shows hypodense areas in apices with −950 UH suggestive of centrilobular and paraseptal emphysema.
We aimed for the identification of COPD endotypes, i.e., groups of similar patients according to a multidimensional evaluation of the disease, including several aspects: clinical, physiological, immunological, pathological, genetic, exposure, prognostic, and different response to treatment6,12.
SPIROMETRIC DEFINITION OF COPD
The demonstration of airflow obstruction is an indispensable criterion for diagnosing COPD, and the gold standard is the finding by spirometry of a reduced forced expiratory volume in 1 second and forced vital capacity (FEV1/FVC) quotient. There is controversy regarding the cut-off point of this quotient that should be used to define obstruction. GOLD defines obstruction as a FEV1/FVC < 0.70, whereas the most common proposed alternative is to use the lower limit of normal (LLN) (the lower 5th percentile), derived from reference values that adjust for age and sex. Table 1 shows some definitions of airflow obstruction used in the past.
Table 1 Spirometric definitions of COPD
| Organization | Criterion to establish obstruction (COPD) |
|---|---|
| GOLD 2017a | Post-BD FEV1/FVC < 0.7 |
| ATS/ERS 2004b | Post-BD FEV1/FVC < LLN |
| ATS 1995c | FEV1/FVC < 5th percentile |
| BTSd | Post-BD FEV1/FVC < 0.7 |
| Alternative | Post-BD FEV1/FEV6 < LLN |
FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity, FEV6: forced expiratory volume in 6 seconds; LLN: lower limit of normal (5th percentile).
aGlobal initiative for chronic obstructive lung disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2017.
bStandards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932-46. ATS; American Thoracic Society. ERS; European Respiratory Society.
dChronic obstructive pulmonary disease in over 16s: diagnosis and management. NICE Clinical Guidelines, 2010. British Thoracic Society. COPD: chronic obstructive pulmonary disease
The 6-s spirometry has been proposed as a simplified alternative to an FVC maneuver because it has the advantage of standardizing the measurement time of the denominator, since the FVC may be measured using good-quality tests at different times and give different results13. The quotient of the FEV1/FEV6 test is nearly equivalent to the FEV1/FVC for COPD diagnosis14,15; however, the former is more reproducible, the required maneuver causes less fatigue, and it is possibly more specific than the FEV1/FVC. There are fewer available reference values for FEV6 and FEV1/FEV6 in comparison with the current gold standards FVC and FEV1/FVC.
GOLD CRITERION VERSUS THE LLN
According to GOLD 2017, an FEV1/FVC quotient of < 0.70 after the bronchodilator test confirms the existence of a persistent airflow limitation and identifies the presence of COPD in patients with compatible symptoms and risks1. This criterion has been employed in numerous clinical assays and is independent of reference values16. However, in healthy persons, FEV1/FVC decreases with age, a situation not considered by that criterion. The fixed cut-off point of 0.70 can cause errors in diagnosis at the extremes of life, resulting in underdiagnosis in young adults (false negatives) and overdiagnosis in older adults (false positives that increase disproportionately with age)17-19. Thus, the prevalence of COPD by that definition is higher than the one estimated by the statistical criterion of <LLN or less than the 5th percentile (20.1% vs. 14.7% in the PLATINO study)20. The high rate of false positives in older adults may cause drug overprescription, adverse effects of medication, excessive use of resources confirming or ruling out the diagnosis, and disease labeling of healthy individuals. The FEV1/FVC < LLN criterion to identify obstruction is more specific, reducing the rate of false-positives; however, the LLN depends on the equation of reference used for the post-bronchodilator values that are being employed. In some populations, it will be necessary to develop spirometry reference values if those currently available do not adequately fit the population18.
The criteria for defining obstruction should influence prognosis and not only be based on a statistical criterion. In this perspective, the cut-off point would be that which identifies an increased benefit or treatment and a deteriorated prognosis without a disproportionate rise in false-positives. The FEV1, per se, has demonstrated to be a strong prognostic indicator, and in groups with reduced FEV1 (such as GOLD stages 2-4), a worse prognosis would be expected. Patients with FEV1/FVC < 0.7 tend to have a lower FEV1 than those with an FEV1/FVC of > 0.7, and adults with FEV1/FVC <LLN an FEV1 lower than individuals with FEV1/FVC < 0.7.
PRE- OR POST-BRONCHODILATOR SPIROMETRY?
The assessment of airflow obstruction should be done with the spirometry performed after the use of bronchodilators, to lower the contribution of asthma and other causes of reversible obstruction. In the PLATINO study, it was demonstrated that the bronchodilator test reduced by 35% (from 21.7% to 14%) the prevalence of COPD by using the FEV1/FVC % < 0.70 criteria, while when using the FEV1/FVC < LLN criterion, the reduction was 37% (from 17.4% to 10.8%)20. The latter provides more certainty in the clinical diagnosis of COPD. Despite what has been discussed, the pre-bronchodilator spirometry is frequently used to assess bronchial obstruction in patients in whom COPD is suspected.
ONE OR MORE SPIROMETRY TESTS FOR THE DIAGNOSIS OF COPD?
In daily clinical practice, the diagnosis of COPD is based on the results obtained in the initial spirometry. To date, there is no recommendation by GOLD guidelines, to repeat the forced spirometry to increase consistency and certainty in the diagnosis. However, the FEV1 and the FVC, their ratio, and all tests, for that matter, vary over time even in healthy subjects. For example, the annual variability reported for the FEV1 and the FVC is of ± 15%. In up to 22% of subjects with a baseline spirometry showing obstruction, their tests normalized during the 1st year of follow-up and, after 2 years, the percentage increased to 24-32%21. These results demonstrate that a longitudinal spirometric evaluation could increase certainty in the diagnosis, mainly in patients with borderline values independently of the reference values and diagnostic criteria employed.
CT SCANNING AND DIFFUSING CAPACITY TO IDENTIFY EMPHYSEMA AND ITS RELATION WITH COPD
Emphysema is defined as an abnormal and permanent dilation of the air spaces that occur distally to the terminal bronchioles and that is accompanied by the destruction of the interalveolar septa, without evidence of fibrosis10. High-resolution CT (HRCT) of the thorax and low-dose tomography of the thorax allow to identify areas of attenuation with <−950 UH, which are consistent with emphysema.
HRCT is the method of choice for diagnosing pulmonary emphysema in vivo22, due to greater spatial resolution compared with the conventional tomography of the thorax23. Quantitative analysis of lung density measured by HRCT permits to evaluate the degree of extension of emphysema; however, this depends to a great extent on the subjective and visual evaluation of the radiologist24. It is now possible to perform a more objective evaluation using tools and software to carry out measurements such as the emphysema index (EI), which defines the relation between the volume of emphysema and total lung volume after a three-dimensional reconstruction. Other indicators include the pixel index (PI), defined as the percentage of pixels with an attenuation of <−900 UH, as well as the EI in expiration (EIex), PI in maximum expiration (PIex), and the pulmonary blood flow (BF)25,26. The sensitivity of the EI in the HRCT is 0.80 (95% confidence interval [CI], 0.74-0.84), while when the tomographic signs (EI, PIex, EIex, and BF) are combined, the sensitivity for detecting emphysema rises up to 0.87 (95% CI, 0.64-0.96)25. In comparison with inspiration, CT measurements in expiration are tightly correlated with airflow obstruction; however, this exposes the patients to additional radiation26.
The use of low-dose CT (20-40 mA) with a slice thickness of 1.25 mm allows for the identification of pulmonary emphysema with accuracy, in addition to permitting the graduation of its extension and correlating it with the histopathological pattern (centrilobular or paraseptal). Likewise, it is possible to identify other typical findings in smoker patients, such as interstitial lung disease, bronchiectasis, and calcification in the coronary arteries or aorta. Low-dose CT can decrease the amount of global radiations for the quantitative evaluation of emphysema, without losing diagnostic value10,26. In a recent study that included smokers with normal spirometry, it was demonstrated that 75% of the participants had emphysema detected in the low-dose CT of the thorax. Although the extension of the emphysema was mild, those findings were associated with a lower quality of life, low DLCO, a greater number of exacerbations in the previous year, and a significant fall in oxygen saturation during the 6-minute walk test (6MWT)10. Despite the benefits of the CT, no guidelines, to our knowledge, recommend its routine use, due to the exposure to radiation and to its considerable cost.
A single-breath carbon monoxide diffusing capacity (DLCO) and a spirometry below LLN values suggest the presence of emphysema27. In patients with pulmonary emphysema, it has been demonstrated that the DLCO is proportional to the extension of the emphysema and correlates with lower resting PaO2, as well as a greater requirement of supplementary oxygen, fewer meters in the 6MWT, and lower maximum exercise capacity28.
RESPIRATORY SYMPTOMS AND COPD
Recently, the relevance of respiratory symptoms has been emphasized since they can predict a poor prognosis, an accelerated decrease in lung function, and exacerbations. Various symptom questionnaires have been developed, including COPD assessment test (CAT) and COPD questionnaire score29. The CAT questionnaire is a sensitive, simple, and quick tool for assessing the respiratory status of COPD patients30. The new GOLD classification incorporates the symptoms (CAT score > 10 and mMRC dyspnea score > 2) and the frequency or severity of exacerbations for therapeutic decisions1. The combination of a long-acting bronchodilator (LABA) and long-acting muscarinic antagonist (LAMA) is recommended for patients classified as GOLD Groups B or C with persistent symptoms after bronchodilator monotherapy with LAMA or LABA31. This combination reduces symptoms and exacerbations compared with LAMA or LABA monotherapy32. However, the classification by symptoms is more unstable and less of a prognostic factor than that based on spirometry33 and, according to current GOLD classification1, can give rise to the prescription of expensive LABA to persons with minimal obstruction, with borderline obstruction, or even to false positives, very frequent in mild COPD such as GOLD stage 1 in older individuals. Respiratory symptoms (cough, phlegm, dyspnea, and wheezing) may have a variety of causes and should be investigated as any other symptom before assuming that they are caused solely by COPD and that will respond to bronchodilators. In population-based studies, those symptoms are associated with smoking, passive smoking, and exposure to occupational agents, as well as with asthma diagnosis, with spirometry abnormalities (obstructive and restrictive), and with self-reported cardiac disease, but a long list of causes is known. The use of questionnaires applied by the clinician, compared to those self-answered by the patient, reduces the number of diagnostic evaluations necessary to identify a COPD patient2,34.
MULTIDIMENSIONAL INDICATORS IN COPD
Multidimensional indicators are increasingly used not only for the diagnosis of COPD but also for prognosis. These indexes have greater prognostic value in comparison with the isolated spirometry measurement35. The body mass index, airflow obstruction, dyspnea score, and exercise (BODE) index (acronym for BODE capacity) has demonstrated to be better than FEV1 for predicting the risk of death due to any cause and due to respiratory causes in patients with COPD36, which is to be expected up to a certain point in that it incorporates multiple domains of the disease and is expected to vary less over time37,38.
DETECTION OF COPD IN THE COMMUNITY AND PRIMARY CARE
In the general population, there is an enormous underdiagnosis of COPD that can rise to 90% when the spirometry definition is used39. The number of spirometry tests conducted in primary care has increased very little, even in developed countries, and in some reports, in only 12.2% of patients with clinical symptoms suggestive of COPD, a spirometry is performed to confirm the diagnosis40-42.
There is a lack of scientific evidence to define the best procedures for the timely detection of COPD, especially in high-risk groups, i.e., case finding. Case finding is a strategy whereby resources are focused on individuals or groups suspected of being at risk for a specific disease, instead of considering the whole population. It implies the active and systematic search for persons at risk instead of waiting for the presentation of the symptoms or signs of active disease. Accumulated smoking (pack-years) is the most important risk factor for airflow obstruction; therefore, the presence of smoking (especially in older men) is a common requisite for selecting individuals for case finding, given that the higher the level of smoking, the prevalence of COPD will also rise in the selected group43,44. The best detection strategy will probably vary according to the country, region, and characteristics of the population and of the health system34. The symptoms and exposures can be explored rapidly with questionnaires, selecting individuals considered at high risk for the spirometry. An intermediate step with a simplified lung function test (peak flow or 6-s spirometry) can increase availability and reduce the number of spirometry tests, a strategy that is, especially, efficient if the objective is to identify moderate-to-severe obstruction39.
A program for the active search of COPD in its pre-clinical stage requires an important assignment of resources; thus, at present, it is considered that, in asymptomatic never smokers or in individuals unexposed to other noxious factors, a screening spirometry is not recommended. Spirometry should be performed preferably on symptomatic patients, older than 40 years of age, with risk factors such as smoking, especially if they smoked > 10 (or 20) pack-years or had other exposure risks such as substantial exposure to biomass smoke or occupational dusts or smokes. In this scenario, up to one in five subjects will have COPD, a number that increases to one of every three subjects in those of higher age and with greater exposure to tobacco. With fewer symptoms, age, or exposures, the number of spirometries performed to identify one individual with airflow obstruction increases progressively and can be cost-ineffective.
In Latin America, the PLATINO study (Proyecto Latinoamericano de Investigación de la Enfermedad Pulmonar Obstructiva, Latin-American Project of Investigation in Obstructive Pulmonary Disease) showed a prevalence of COPD of 14.3% with the GOLD criterion (FEV1/FVC < 0.70), of whom nearly 90% had no medical diagnosis, that is, 90% of patients with COPD did not know that they had the disease33,45. The main factors for an underdiagnosis of COPD were a younger age, mild obstruction, fewer respiratory symptoms, and importantly, the lack of a spirometry test42.
In the PUMA study (prevalence study and regular practice, diagnosis, and treatment, among general practitioners in populations at risk of COPD in Latin America), at-risk subjects were included if they were ≥ 40 years old, current or ex-smokers (≥ 10 pack-years), and/or with exposure to biomass smoke (wood or coal, for cooking or heating; exposure ≥ 100 h/year). The COPD prevalence in this study was 20.1% and 14.7% using post-BD FEV1/FVC < 0.70 and LLN definitions, respectively43.
CONCLUSION
The current definition of COPD includes a post-bronchodilator spirometry in subjects with exposures and risk factors, although the cut-off point to define obstruction varies, generating definitions with more or less specificity. While the present-day guidelines recommend a single spirometry test, the variability of the latter, particularly in borderline tests, requires an observation over time and the performance of repeated tests. The FEV1/FEV6 index is more reliable than the FEV1/FVC, specifically when groups with spirometries with a different expiratory time are compared.











 nueva página del texto (beta)
nueva página del texto (beta)


