Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de investigación clínica
versão On-line ISSN 2564-8896versão impressa ISSN 0034-8376
Rev. invest. clín. vol.58 no.5 Ciudad de México Set./Out. 2006
Artículo original
The Janus Kinase 2 (JAK2) V617F mutation in hematological malignancies in México
La mutación V617F del gen de la cinasa JAK2 en padecimientos hematológicos malignos en México
Guillermo J. Ruiz–Argüelles,*,** Javier Garcés–Eisele,**,**** Virginia Reyes–Núñez,** Guillermo J. Ruiz–Delgado,**** Morelis Navarro–Vázquez,* Martha L. González–Carrillo**
* Centro de Hematología y Medicina Interna de Puebla.
** Laboratorios Clínicos de Puebla.
*** Universidad de las Americas de Puebla.
**** Hospital Universitario de Monterrey.
Correspondence and reprint request:
Guillermo J. Ruiz–Argüelles MD,
Dirección General. Centro de Hematología y Medicina Interna de Puebla.
8B Sur 3710.
72530, Puebla, Pue., México
Phone: + 52 (222) 243 8100 Fax: + 52 (222) 243 8428
E–mail: gruizl@clinicaruiz.com
Recibido el 22 de febrero de 2006.
Aceptado el 13 de junio de 2006.
ABSTRACT
A new mutation (V617F) affecting the JAK2 gene has been recently described as acquired in patients with myeloproliferative disorders and other myeloid malignancies. Using an amplification refractory mutation system, we investigated this mutation in 70 Mexican mestizo patients with hematological malignancies: 28 cases of acute lymphoblastic leukemia, 17 cases of Phi–positive chronic myelogenous leukemia, 8 patients with acute myelogenous leukemia, 6 patients with chronic lymphocytic leukemia, 6 patients with polycythemia vera (PV), two patients with essential thrombocythemia (ET), one patient with hypereosinophilic syndrome one patient with primary myelofibrosis (MF) and one patient with chronic myelomonocytic leukemia. The mutation was identified in 4 of 6 patients with PV, in one of 2 patients with ET and in the patient with MF. Our data add to the observation that the JAK2 V617F mutation seems to be rather uncommon in myeloid malignancies other than the classic BCR/ABL negative MPD.
Key words. JAK2 V617F mutation. Myeloproliferative. Disorders. México.
RESUMEN
Se ha descrito una nueva mutación (V617F) que afecta al gen de la cinasa JAK2 en pacientes con padecimientos mieloproliferativos y otras neoplasias mieloides. Empleando un sistema de amplificación de mutaciones refractarias y reacción en cadena de la polimerasa, investigamos esta mutación en 70 pacientes mestizos mexicanos con neoplasias hematológicas malignas: 28 casos de leucemia aguda linfoblástica, 17 casos de leucemia granulocítica crónica BCR/ABL (+), ocho casos de leucemia aguda mieloblástica, seis casos de leucemia linfocítica crónica, seis casos de policitemia vera (PV), dos casos de trombocitosis primaria (TP), un caso de síndrome hipereosinofílico primario y un caso de mielofibrosis primaria (MF) y un caso de leucemia mielomonocítica crónica. La mutación se identificó en cuatro de seis pacientes con PV, en uno de dos pacientes con TP y en el paciente con MF. Estos datos confirman que esta mutación es infrecuente en neoplasias hematológicas mieloides diferentes a los síndromes mieloproliferativos malignos negativos al BCR/ABL; es probable que esta mutación se convierta en el marcador molecular de la PV.
Palabras clave. Mutación JKZ V617F. Síndromes mieloproliferativos. México.
INTRODUCTION
Janus (ianua), the Roman god of gates and doors, of beginnings and endings, is represented with a double–faced head, each looking in opposite directions; he is also called bifrons (two fronts or two faces). Protein kinases (PK) are enzymes that catalyze protein phosphorylation, whereas protein phosphatases do the opposite: regulate PK activity through protein dephosphorylation. Protein–tyrosine kinases (PTK) are PK that catalyze the transfer of the γ–phosphate group of adenosine triphosphate (ATP) to the hydroxyl groups of specific tyrosine residues in signal transduction molecules. In humans, the Janus PTK family (JAKs), has two similar domains facing in opposite directions –hence its name of roman origin– and contains four members: JAK1, JAK2, JAK3 and TYK2. JAKs phosphorylate signal transducers and activators of transcription (STATs) simultaneously with other phosphorylations required for activation.1
Nowell and Hungerford2 described in 1960 the first disease–specific cytogenetic marker, the Philadelphia chromosome (Phi) in chronic myelogenous leukemia (CML). Since then, other molecular markers of leukemia and myeloproliferative disorders (MPDs) have been described.3 A new mutation (V617F) affecting the JAK2 gene has been recently described as acquired in a large proportion of patients with MPDs and other myeloid disorders.3,4 We report herein the results of looking for the JAK2 V617F mutation in a group of 70 Mexican mestizo patients with different hematologic malignancies.
MATERIAL AND METHODS
Patients
Patients with hematological malignancies studied at Centro de Hematología y Medicina Interna de Puebla after March 2005 were prospectively accrued in the study; in addition, DNA samples from our bank were also studied. The diagnosis and classification of leukemia were done according to conventional criteria;5 patients were studied, treated and followed by one of us. As normal controls 150 healthy blood donors were studied. The operational definition of mestizo, recently published by Pons–Estel et al.6 was employed to select the patients: Individuals born in Latin America who had both Amerindian and white ancestors.
Analysis of the JAK2 V617F mutation
An amplification refractory mutation system (ARMS) method was used according to Baxter et al.1 Briefly, genomic DNA was isolated from peripheral blood leukocytes according to standard procedures. In a multiplex format, the mutation was detected with the help of alíele specific primers (203 bp) and the complete exon 12 was amplified as an internal amplification control (364 bp), taking care to not exceed 0.05µg of DNA per 50 µl amplification reaction. Amplification products were analyzed after electrophoresis on 4.5% polyacrylamide gels (Figure 1).
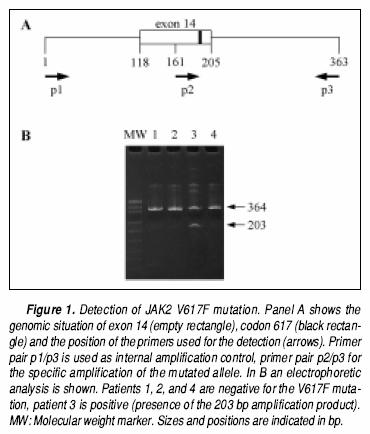
RESULTS
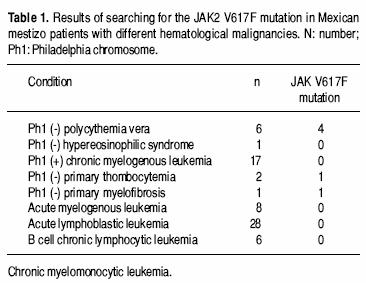
Of the 70 samples with a known diagnosis of a hematological malignancy, there were six cases positive for the JAK2 V617F mutation. The table 1 shows these results.
The patient with primary myelofibrosis was allografted from his HLA identical sibling; interestingly, the detection of the V617F affecting the JAK2 gene has diminished substantially as the patient becomes a chimera.
DISCUSSION
Chronic myeloproliferative diseases are clonal hematopoietic stem cell disorders characterized by proliferation of one or more myeloid cell lineages in the bone marrow and increased numbers of mature and immature cells in the peripheral blood.3,8–9 CMPDs include polycythemia vera (PV), essential thrombocythemia (ET), idiopathic myelofibrosis (MF) and CML, plus rarer subtypes such as chronic neutrophilic leukemia, hypereosinophilic syndrome, and chronic eosinophilic leukemia. These diseases overlap with myelodysplastic/myeloproliferative diseases such as atypical CML and chronic myelomonocytic leukemia, in which proliferation is accompanied by dysplastic features or ineffective hematopoiesis in other lineages.3,10 Although there are stringent diagnostic criteria for CMPDs subtypes, precise categorization remains a subject of debate and furthermore, it can be difficult to differentiate some cases from reactive disorders. Only CML is characterized by a pathognomonic molecular marker, the BCR–ABL fusion, and the primary abnormalities driving excess proliferation in most other cases have been obscure. Several lines of evidence have implicated aberrant PTK signaling as the root cause of some CMPDs. BCR–ABL itself is a constitutively active tyrosine kinase that is believed to be the primary, and probably the only, driving force behind chronic–phase CML.3'10 Other gene fusions have been identified in rare cases of CMPD's that involve the tyrosine kinases PDGFRA, PDGFRB, FGFRI, and JAK2.3,10 In addition, the KIT receptor is activated by a point mutation in the majority of cases of systemic mastocytosis. To investigate the molecular pathogenesis of CMPDs, mutation screen studies for genes encoding tyrosine kinases and down stream signaling components have been conducted.3,10
The molecular markers of several hematological malignancies may have a different distribution worldwide. We have shown that in México, the prevalence of the bcrl subtype of the PML/RARa fusion gene is significantly higher than that informed in Caucasians and similar to that in Asians,11 whereas the distribution of the bcr subtypes of the BCR/ABL fusion gene is similar to that described in Caucasian.12 These "molecular" differences may account for some of the differences in the prevalence of several hematologic malignancies in certain populations, such as the high prevalence of the promyelocytic leukemia in México.13
CMPDs are less frequent in México than in Caucasian populations;9 of the CMPDs, PV is the less frequent one in Mexican mestizos.9 In this study, we have found a similar prevalence of the JAK2 V617F mutation in patients with different hematological malignancies. The JAK2 V617F mutation could eventually become the molecular marker of PV, but it also occurs in other myeloproliferative disorders;3,4,10 we found the mutation in 4 of 6 patients with PV, in one of 2 patients with ET and in the patient with MF, while all patients positive for the Philadelphia chromosome were negative as published previously.10,14 Our data add to the observation that the JAK2 V617F mutation seems to be rather uncommon in myeloid malignancies other than the classic BCR/ABL negative MPD and that the V617F–negative MPD are likely to reflect mutations in other molecules that modulate the JAK/STAT pathway, or mutations in different signaling pathways.14 Despite the fact that the JAK2 V617F is not present in all cases of PV,15 it is possible that it will eventually become the molecular marker of this disease;3,4,14–16 on the other hand, and at the very least, this newly identified molecular lesion will most likely form the basis of a new classification of the CMPDs15 and of the definition of the "molecular remission" of these diseases.17
The identification of molecular markers of diseases may have therapeutic implications. It is a tantalizing prospect that one might be able to modulate selected JAK/STAT–mediated cellular signals by inhibiting JAK kinase activity in order to achieve a positive therapeutic outcome; while current data suggest no therapeutic use for JAK1 and TYK2 inhibition, JAK2 inhibition seems a promising but not definitively tested mechanism for treatment of leukemia.1,15 Enhanced protein tyrosine kinase (PTK) activity correlates with the development of cancer and other proliferative diseases. The hypothesis that PTK inhibitors may be of value in the treatment of cancer led to the systematic synthesis of selective tyrosine phosphorylation inhibitors (tyrphostins) that show in vitro and in vivo anticancer activity. Research efforts in the development of tyrphostins such as AG 957, AG 1112, and AG 1318 have been done; other tyrphostins are AG 1478 and RG 13022, which are both epidermal growth factor receptor kinase inhibitors; AG 490, a JAK2 kinase inhibitor; AG 1296, a PDGFR kinase inhibitor, and STI 571 (imatinib), already in clinical use.1 It is clear that a better understanding of the molecular mechanisms of the diseases will lead into a better design of specific treatments.
REFERENCES
1. Thompson JE. JAK protein kinase inhibitors. Drug News Perspect 2005; 18: 305–10. [ Links ]
2. Nowell PC, Hungerford DA. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst 1960; 25: 85–109. [ Links ]
3. Tefferi A, Gilliland G. The JAK2 V617F tyrosine kinase mutation in myeloproliferative disorders: Status report and immediate implication for disease classification and diagnosis. Mayo Clin Proc 2005; 80: 947–58. [ Links ]
4. James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. Constantinescu SN, Casadevall N, Vainchenker W. A unique clonal JAK2 mutation leading to constitutive signallig causes polycythemia vera. Nature 2005; 434: 1144–8. [ Links ]
5. Ruiz–Argüelles GJ, McArthur JR. Leucemias agudas. En: Fundamentos de Hematología, 3rd. edition. Ruiz–Argüelles GJ (Ed.). México: Editorial Médica Panamericana; 2003, pp. 225–45. [ Links ]
6. Pons–Estel BA, Catoggio LJ, Cardiel MH, Soriano ER, Gentiletti S, Villa AR, Abadi I, Caeiro F, Alvarellos A, Alarcón–Segovia D. The GLADEL multinational Latin American prospective inception cohort of 1214 patients with systemic lupus erythematosus. Ethnic and disease heterogeneity among "Hispanics". Medicine 2004; 83: 1–17. [ Links ]
7. Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, Scott MA, Erber WN, Green AR. Cancer Genome Project. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005; 365: 1054–61. [ Links ]
8. Labardini–Méndez J. Síndromes mieloproliferativos. En: Fundamentos de Hematología, 3rd. edition. Ruiz–Argüelles GJ (Ed.). México: Editorial Médica Panamericana; 2003, pp. 261–78. [ Links ]
9. Ruiz–Argüelles GJ, López–Martínez B, Lobato–Mendizábal E, Ruiz–Delgado GJ. An addition to geographic hematology: Chronic myeloproliferative diseases are infrequent in Mexican Mestizos. Int J Hematol 2002; 75: 499–502. [ Links ]
10. Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood 2005; 106: 2162–8. [ Links ]
11. Ruiz–Argüelles GJ, Garcés–Eisele J, Reyes Núñez V, Gómez–Rangel JD, Ruiz–Delgado GJ. More on geographic hematology: The breakpoint cluster regions of the PML/RARa fusion gene in Mexican mestizo patients with promyelocytic leukemia are different from those in Caucasians. Leuk Lymphoma 2004; 45: 1365–8. [ Links ]
12. Ruiz–Argüelles GJ, Garcés–Eisele J, Reyes–Núñez V, Ruiz–Delgado GJ. Frequencies of the breakpoint cluster region types of the BCR/ABL fusion gene in Mexican mestizo patients with chronic myelogenous leukemia. Rev Invest Clin 2004; 26: 609–14. [ Links ]
13. Ruiz–Argüelles GJ. Promyelocytic leukemia in Mexican mestizos. Blood 1997; 89: 348–9. [ Links ]
14. Scott LM, Campbell PJ, Baxter J, Todd T, Stephens P, Edkins S, Wooster R, Stratton MR Futreal PA, Green AR. The V617F JAK2 mutation is uncommon in cancers and in myeloid malignancies other than the classic myeloproliferative disorders. Blood 2005; 106: 2920–1. [ Links ]
15. Goldman JM. A unifying mutation in chronic myeloproliferative disorders. N Engl J Med 2005; 352: 1744–6. [ Links ]
16. Mesa RA, Powell H, Lasho T, deWald GW, McClure R, Tefferi A. A longitudinal study of the JAK2 V617F mutation in myelofibrosis with myeloid metaplasia: Analysis at two time points. Haematologica 2006; 91: 415–6. [ Links ]
17. Ruiz–Argüelles GJ, Garcés–Eisele J, Reyes–Nuñéz V, Ruiz–Delgado GJ, Rosilio C, Camoriano JK. Clearance of the Janus Kinase 2 (JAK2) V617F mutation after allogenic stem cell transplantation in a patient with myelofibrosis with myeloid metaplasis. Am J Hematol 2006; 58(5): 458–61. [ Links ]














