Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de investigación clínica
versão On-line ISSN 2564-8896versão impressa ISSN 0034-8376
Rev. invest. clín. vol.57 no.1 Ciudad de México Jan./Fev. 2005
Artículo original
Sentinel lymph node biopsy in colorectal cancer: A pilot study
Biopsia de ganglio centinela en cáncer colorrectal: Estudio piloto
Heriberto Medina–Franco,* Takeshi Takahashi,** Gabriel F. González–Ruiz,* Jazmin De–Anda,*** Liliana Velazco**
* Division of Surgery, Section of Surgical Oncology,
** Colorectal Surgery and
*** Pathology. Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán.
Correspondence and reprint request:
Heriberto Medina–Franco, M.D.
Division of Surgery
National Institute of Medical Sciences and Nutrition Salvador Zubirán.
Vasco de Quiroga No. 15. Tlalpan.
14000, Mexico City.
Phone: 55739321. Fax: 55739321.
E–mail: herimd@hotmail.com
Recibido el 26 de febrero de 2004.
Aceptado el 23 de noviembre de 2004.
ABSTRACT
Background. Although sentinel lymph node biopsy technique is the gold standard in the management of malignant melanoma and is gradually replacing conventional axillary dissection in breast cancer, its use in colorectal cancer is still controversial. The objective of this study is to demonstrate the feasibility and safety of sentinel node biopsy in the management of colorectal carcinoma.
Methods. Consecutive patients with colorectal carcinoma without preoperative evidence of nodal or distant metastatic disease were included. Intraoperative subserosal injection of 1mL of isosulfan blue (Lymphazurin ®) was performed around the tumor in cases of colon cancer and ex–vivo infiltration was used for rectal cancer after resection was completed. Blue stained nodes were dissected and submitted for routine pathology exam. If nodes were deemed negative for neoplasm, immunohistochemistry for cytokeratin was performed. The specimen and non–stained nodes were resected and processed in the usual fashion. Sensitivity and negative predictive value were calculated and adverse effects to the blue dye were registered.
Results. Ten patients were included with at least one sentinel lymph node identified in each. Mean number of sentinel and non–sentinel lymph nodes were 2.5 and 15.6 per patient, respectively. The sensitivity and negative predictive value of the sentinel node after immunohistochemistry were both 100%. There were no adverse effects caused by the dye.
Conclusions. Sentinel lymph node biopsy technique in colorectal cancer is feasible, has a high diagnostic accuracy and is harmless.
Key words. Sentinel node. Colon cancer. Rectal cancer.
RESUMEN
Introducción. A pesar que la técnica de biopsia del ganglio centinela es el estándar de oro en el manejo del melanoma maligno y que gradualmente está reemplazando la disección axilar convencional en el cáncer mamario, existe controversia en el uso de esta técnica en cáncer colorrectal. El objetivo de este estudio es demostrar la factibilidad y seguridad de la técnica del ganglio centinela en el manejo del carcinoma colorrectal.
Métodos. Pacientes consecutivos con diagnóstico de carcinoma colorrectal sin evidencia preoperatoria de metástasis ganglionares o distantes fueron incluidos en el estudio. Se realizó inyección subserosa intraoperatoria de 1 mL de azul de isosulfán (Lymphazurin ®) alrededor del tumor en los casos de cáncer colónico e infiltración ex vivo fue empleada en casos de cáncer rectal una vez finalizada la resección. Los ganglios teñidos de color azul fueron disecados y enviados para examen rutinario de patología. Si los ganglios eran negativos para neoplasia se estudiaban mediante inmunohistoquímica para citoqueratinas. Los ganglios no teñidos fueron resecados y procesados de manera rutinaria. Se calcularon la sensibilidad y el valor predictivo negativo y se registraron los efectos nocivos del colorante azul.
Resultados. Se incluyeron diez pacientes, encontrándose por lo menos un ganglio centinela en cada uno de ellos. El promedio de ganglios centinela y no–centinela identificados por paciente fue de 2.5 y 15.6, respectivamente. Tanto la sensibilidad como el valor predictivo negativo del ganglio centinela después de la tinción con inmunohistoquímica fueron del 100%. No se registraron efectos adversos causados por el colorante.
Conclusiones. El uso de la técnica de biopsia del ganglio centinela en cáncer colorrectal es factible, tiene alta exactitud diagnóstica y es inocua.
Palabras clave. Ganglio centinela. Cáncer de colon. Cáncer de recto.
The presence of lymph node metastasis is recognized as the single most important prognostic factor in colorectal cancer (CRC). Besides its importance in determining prognosis, the lymph node status is also used as the primary indicator of systemic disease spread and the rationale for postoperative adjuvant chemotherapy.1–3 Still, approximately 30% of patients diagnosed with early CRC (Stages I or II of the American Joint Committee on Cancer –AJCC–) develop systemic disease. This implies that the subgroup of patients with early CRC harbors minimal but significant amount of occult disease that is not detected by current techniques. Ultrastaging techniques such as multiple level sectioning, immunohistochemical staining, and polymerase chain reaction assays demonstrate the presence of lymph node micrometastases in a significant proportion of patients whose nodes are negative by routine hematoxylin and eosin staining.4–6 Although the etiology of recurrence in these patients is likely to be multifactorial, several retrospective studies have established a poorer prognosis in those patients harboring nodal micrometastasis demonstrated by microstaging techniques.4,5 Because ultrastaging techniques are labor intensive and expensive, it has been proposed to focus the efforts to the sentinel lymph node. The sentinel lymph node is the first node to receive the lymphatic drainage of a certain region, and its role in melanoma and breast cancer is well defined.7
In addition, proximally situated locoregional lymph nodes metastases or involvement of the apical node are associated with poorer prognosis.8,9 Similarly with melanoma10 and breast cancer,11 there has been no strong evidence that extended lymph node dissection in rectal cancer has any impact on survival or local recurrence,12,13 therefore suggesting that lymph node metastasis are indicators of systemic disease rather than governors of survival.14 However, it is important to be able to stage the disease accurately to define those who may benefit from adjuvant therapy.15 Thus, in CRC, lymphatic mapping and the sentinel node technique are used to improve staging by means of focused pathologic ultrastaging examination of the sentinel nodes (the lymph nodes most likely to contain the earliest metastases) and to identify lymphatic routes outside the usual lymphatic drainage encompassed in routine lymphadenectomy.
The objective of the present study was to establish the feasibility and safety of the sentinel lymph node biopsy technique in CRC and to determine its diagnostic accuracy.
METHODS
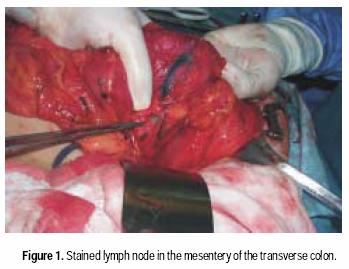
Between August 2001 and April 2002, consecutive patients undergoing resection for clinically localized CRC at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán in Mexico City, were enrolled in a prospective study of sentinel node mapping. Informed consent was obtained preoperatively from all patients. All patients were staged before surgery with chest X ray, routine lab measures and CT scan of the abdomen and pelvis. Patients with metastatic disease were not included in the study. All patients were approached via conventional open colon or rectal resection techniques. Laparotomy and routine operative exploration of the abdomen were performed. Patients with evidence of metastatic disease or enlarged lymph nodes suggestive of metastatic involvement were not included in the present study. After this evaluation and once resectability was determined, the involved segment of the colon was mobilized. One milliliter of isosulfan blue (Lymphazurin ®, US Surgical Corp, Ben Venue Laboratories, Inc., Bedford, Ohio) was carefully injected subserosally into four quadrants around the periphery of the tumor using a tubeculin syringe. The dye traveled from the injection site along the lymphatic vessels to the sentinel lymph node(s) typically within five minutes (Figure 1). Each blue stained node was carefully dissected and sent to pathology lab as sentinel lymph node. After this maneuver, colectomy was performed in the standard fashion, including all nodes in the mesenteric resection specimen. The specimen was submitted for pathologic analysis.
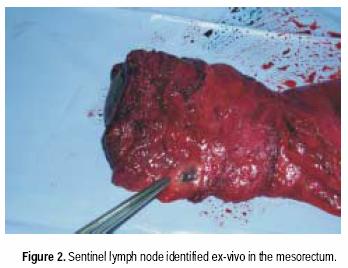
For rectal cancer cases, laparotomy and routine exploration was performed. Rectal resection with total mesorectal excision was routinely performed in all cancers 10 cm or less from the anal verge. After resection of surgical specimen, lymphatic mapping was performed with the specimen ex vivo, modifying the technique described by Wong et al.16 Within 30 minutes of resection, using a tuberculin syringe, four separate subserosal injections of 0.25 cc isosulfan blue (Lymphazurin ®), were performed in four quadrants around the tumor. The injection sites were gently massaged for approximately 2 to 5 minutes. The mesentery was then examined by gently incising the overlying endorectal fascia, the mesenteric fat was bluntly separated, and using a meticulous blunt dissection, blue lymphatic channels were identified and subsequently traced though adipose tissue of the mesentery to a blue–stained lymph node (Figure 2). These blue–stained nodes were then individually harvested and submitted for histologic examination.
Pathologic analysis entailed routine microscopic analysis of the tumor, margins, and all lymph nodes with hematoxylin and eosin staining. Lymph nodes were manually dissected from the mesenteric fat. All nodes identified were dissected, and a single section was examined with hematoxylin and eosin staining. If results were negative, each sentinel node was examined by a focused technique originally developed for the examination of sentinel lymph nodes draining primary breast carcinomas.17 The pathologist sectioned each sentinel node in slices no thicker than 2 to 3 mm. One section for each level was stained with hematoxylin and eosin and another with cytokeratin immunohistochemistry. Immunohistochemical stains were interpreted according to strict criteria that required strong immunoreactivity combined with microanatomic and cytologic features compatible with CRC. A false negative sentinel node was defined as sentinel node that contained no tumor cells when one or more non–sentinel nodes in the specimen were positive for tumor. Sensitivity, and negative predictive value of the sentinel node technique were calculated and adverse reaction to isosulfan blue were carefully recorded.
RESULTS
The study group consisted of 10 patients: 3 male and 7 female, whose average age was 69 years (range 42 to 90 years). Primary tumors were in the right colon (n =5), transverse colon (n = 1), left colon (n = 1), or rectum (n =3). According to Dukes' staging, one patient presented with stage A, 3 patients B1 and 6 patients B2 (one Tl, 3 T2 and 6 T3, according to the AJCC criteria). Two cases were classified as undifferentiated tumors and four cases each were moderately or well differentiated tumors. All colon cases were done with an in vivo technique and rectal cancers with ex vivo technique.
Lymphatic mapping successfully demonstrated at least one sentinel lymph node in all cases (100%). An average of 2.5 sentinel lymph nodes were identified in each case (range 1 to 7), and the average number of non–sentinel nodes harvested from each CRC specimen was 15.6 (range 5 to 42). The total number of examined nodes was 181 and 25 (13.8%) were considered sentinel nodes. In all cases, the sentinel node was near the primary tumor or in the expected field of routine lymphadenectomy. We did not identify aberrant lymphatic drainage in any case.
Identified sentinel lymph nodes accurately reflected the status of the nodal basin after immunohisto–chemical staining in 10 of 10 patients (100%). There was one rectal case, in whom the sentinel node was reported as H&E negative and two non–sentinel nodes were reported with metastatic disease. After IHC staining, micrometastasis in the sentinel node was identified. One additional right colon cancer, with all nodes negative for metastatic disease by H&E including sentinel lymph node, after IHC staining, was found to have micrometastasis in the sentinel node. Overall, 3 out of 10 patients had metastatic disease by H&E and one additional patient was found to have micrometastatic disease in the sentinel node. In summary, the sensitivity and negative predictive value of the sentinel node after IHC staining were both 100%. There were no adverse reactions reported due to isosulfan blue injection in any case. The only symptom reported in all in vivo cases were the presence of green urine 24 to 36 hours after the surgical procedure.
DISCUSSION
Standard pathologic methods of harvesting and studying lymph nodes from the resected colon specimens vary from one institution to another. Sampling error may lead to understaging and can occur when informative lymph nodes are not harvested from examination. Special harvesting methods may reveal small nodes that are often missed; these small occult nodes can frequently contain metastatic deposits.18 Additionally, routine histologic lymph node examination techniques may also lead to understaging. Routinely, lymph nodes are bisected and only one or two sections are examined by hematoxylin and eosin staining, leaving more than 90% of the node unexamined. However, special pathologic examination of multiple section of the numerous lymph nodes revealed by these special techniques is expensive and time consuming. A method to select the ultrastaging examination of the lymph nodes most likely to harbor the earliest evidence of metastasis is desirable from both a logistical and economic standpoint. Application of lymphatic mapping and the sentinel lymph node technique facilitates such a focused pathologic examination. Unlike in melanoma and breast cancer, the application of sentinel lymph node technique in CRC is not intended to limit unnecessary lymphadenectomy. Actually, some authors have reported than in small number of patients, the presence of aberrant lymphatic drainage, lead them to perform more aggressive resection, encompassing in the lymphadenectomy field, areas not routinely included in the dissection.19 In our study we did not find any case of aberrant lymphatic drainage.
Our study agree with others, regarding the high rate of lymph node identification in CRC,16,19,20 whereas the reported rates of sentinel node identification varies from 88 to 100%. Most authors concur that sentinel nodes are more difficult to identify in rectal cancers,19 so ex–vivo techniques used in the present study as previously described,16 have proved to improve the rate of sentinel node identification in these cases. The accuracy of the sentinel node technique in CRC has varied from 100% in our present paper, 95% in a large study by Wood et al., 19 and a false negative rate of 45% in a small study by Merrie et al.20. One recently published study using combination of probe and dye–directed lymphatic mapping in early CRC reported an accuracy of 93.8% in 49 patients.21
Preliminary studies have demonstrated the prognostic significance of the molecular detection of tumor DNA or RNA in the blood or bone marrow of patients with colon cancer.22,23 Other investigators have hypothesized that molecular tumor genoty–ping will someday provide important prognostic and clinically useful information.24 Although these technologically advanced and specialized assays are likely to be extremely informative in the future, currently they are not generally available and reproducible in a clinical setting. At present, lymph node status remains the single most important and practical prognostic factor in CRC.
Although the prognostic importance of nodal status by routine hematoxylin and eosin staging has been proved, the prognostic significance of nodal micrometastatic disease remains unclear. Whereas a number of small retrospective studies have failed to demonstrate a difference in survival between those with micrometastatic disease and those with node–negative disease25,26 other similarly small studies have demonstrated a significant survival advantage in those patients without evidence of micrometastatic disease.45 In our study we detected one patient with lymph node metastases detected only by immunohistochemical staining for cytokeratins. A prospective trial including a standardized focused examination of the sentinel lymph node seems to be the method most likely to separate accurately those with early metastatic disease from those who are truly node negative, and to determine the prognostic value of nodal micrometastasis in CRC.
Although a significant proportion of patients with early–stage (AJCC stage I or II) CRC develop systemic disease, treatment of this group of patients with chemotherapy is currently controversial. To date, no prospective randomized trial has specifically addressed whether chemotherapy provides a survival advantage over surgery alone in stage I or stage II colon cancer. Two published meta–analyses have attempted to provide insight into this question by pooling patients with Dukes' stage B colon cancer from available adjuvant therapy trials. In the United States, the National Surgical Adjuvant (NSABP) analyzed patients with Dukes' B colon cancer included in its C–01, C–02, C–03, and C–04.27 The group concluded that patients with Dukes' B colon cancer who are treated with chemotherapy seemed to benefit and that chemotherapy should be routinely considered in these patients. Besides the inherent weakness of this study as a meta–analysis, it has also been criticized because none of its four trial investigated the standard adjuvant regimen of 5–fluoruracil/leucovorin versus surgery alone. A separate international multicenter group published the result of a meta–analysis (IMPACT B–2) that specifically pooled patients with Dukes' B2 colon cancer from five separate trials in which the standard treatment was used.28 This study found no improvement in event–free or overall survival with adjuvant therapy. Thus, the clinical benefit of adjuvant chemotherapy in early–stage CRC remains unclear.
It is likely that certain subgroup of patients with early–stage CRC might indeed benefit from adjuvant therapy. In the future, certain phenotypic and genotypic/molecular characteristics of the primary tumor and lymph nodes might provide a mean to stratify patient risk of recurrence after resection of early–stage CRC. Currently, the sentinel node technique seems to be the best way to identify the group of patients with early metastatic disease.
In conclusion, sentinel lymph node mapping can be performed in carcinoma of the colon and rectum safely and with great degree of success. Although the sentinel node technique in CRC is not intended to minimize the extent or morbidity of lymphadenectomy as in melanoma or breast cancer, it facilitates a focused pathologic examination of the lymph nodes most likely to harbor early metastases, the sentinel nodes. There is a need to determine the prognostic significance of nodal micrometastases in colorectal carcinoma.
REFERENCES
1. Fielding LP, Phillips RK, Fry JS, Hittinger R. Prediction of outcome after curative resection for large bowel cancer. Lancet 1986; 2: 904–7. [ Links ]
2. Cohen AM, Tremiterra S, Candela F, Thaler HT, Sigurdson ER. Prognosis of node–positive colorectal cancer. Cancer 1991; 67: 1859–61. [ Links ]
3. Malassagne B, Valleur P, Serra J, et al. Relationship of apycal node involvement to survival in resected colon carcinoma. Dis Colon Rectum 1993; 36: 645–53. [ Links ]
4. Hayashi N, Ito I, Yanagisawa A, Kato Y, Nakamori S, Imaoka S, Watanabe H, Ogawa N, Nakamura Y. Genetic diagnosis of lymphnode metastasis in colorectal cancer. Lancet 1995; 345: 1257–9. [ Links ]
5. Liefers GJ, Cleton–Jansen AM, Van de Velde CJ, Hermans J, Van Krieken JH, Cornelisse CJ, Tollenaar RA. Micrometastasses and survival in stage II colorectal cancer. N Engl J Med 1998; 339:223–8. [ Links ]
6. Calaluce R, Miedema BW, Yesus YW. Micrometastasis in colorectal carcinoma: a review. J Surg Oncol 1998; 67: 194–202. [ Links ]
7. Krag ND. Minimal access surgery for staging regional lymph nodes: the sentinel node concept. Curr Prob Surg 1998; 35: 953–1017. [ Links ]
8. Shida H, Ban K, Matsumoto M, et al. Prognostic significance of location of lymph node metastases in colorectal cancer. Dis Colon Rectum 1992; 35: 1046–50. [ Links ]
9. Tang R, Wang JY, Chen JS, et al. Survival impact of lymph node metastasis in TNM stage III carcinoma of the colon and rectum. J Am Coll Surg 1995; 180: 705–12. [ Links ]
10. Balch CM, Soong SJ, Bartolucci AA, et al. Efficacy of an elective lymph node dissection of 1 to 4 mm thick melanomas for patients 60 years of age and younger. Ann Surg 1996; 224: 255–66. [ Links ]
11. Greco M, Agresti R, Raselli R, Giovanazzi R, Veronesi U. Axillary dissection can be avoided in selected breast cancer patients: analysis of 401 cases. Anticancer Res 1996; 16: 3913–17. [ Links ]
12. Pezin ME, Nicholls RJ. Survival after high or low ligation of the inferior mesenteric artery during curative surgery for rectal cancer. Ann Surg 1984; 200: 729–33. [ Links ]
13. Surtees P, Ritchie JK, Phillips RK. High versus low ligation of the inferior mesenteric artery in rectal cancer. Br J Surg 1990; 77: 618–21. [ Links ]
14. Cady B. Lymph node metastases. Indicators, but not governors of survival. Arch Surg 1984; 119: 1067–72. [ Links ]
15. NIH Consensus Conference. Adjuvant therapy for patient with colon and rectal cancer. JAMA 1990; 264: 1444–50. [ Links ]
16. Wong JA, Steinman S, Calderia C, Bowies J, Namiki T. Ex vivo sentinel node mapping in carcinoma of the colon and rectum. Ann Surg 2001; 233: 515–21. [ Links ]
17. Turner RR, Ollila DW, Stern S, Giuliano AE. Optimal histopathologic examination of the sentinel node for breast cancer staging. Am J Surg Pathol 1999; 23: 263–7. [ Links ]
18. Koren R, Siegal A, Klein B, Halpern M, Kyzer S, Veltman V, Gal R. Lymph node–reveling solution: simple new method for detecting minute lymph nodes in colon carcinoma. Dis Colon Rectum 1997; 40: 407–10. [ Links ]
19. Wood TF, Nora DT, Morton DL, Turner RR, Rangel D, Hutchinson W, Bilchik AJ. One hundred consecutive cases of sentinel lymph node mapping in early colorectal carcinoma: detection of missed micrometastasis. J Gastrointest Surg 2002; 6: 322–30. [ Links ]
20. Merrie AEH, Van Rij AM, Phillips LV, Rossaak JI, Yun K, Mc–Call JL. Diagnostic use of the sentinel node in colon cancer. Dis Colon Rectum 2001; 44: 410–7. [ Links ]
21. Trocha SD, Nora DT, Saha SS, Morton DL, Wiese D, Bilchik AJ. Combination probe and dye–directed lymphatic mapping detects micrometastases in early colorectal cancer. J Gastrointest Surg 2003; 7: 340–6. [ Links ]
22. Mori M, Mimori K, Ueo H, Tsuji K, Shiraishi T, Barnard GF, Sugimachi K, Akiyoshi T. Clinical significance of molecular detection of carcinoma cells in lymph nodes and peripheral blood by reverse transcription–polymerase chain reaction in patients with gastrointestinal or breast carcinomas. J Clin Oncol 1998; 16: 128–32. [ Links ]
23. Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S. Significant detection of circulating cancer cells in blood by reverse transcriptase–polymerase chain reaction during colorectal cancer resection. Ann Surg 2000; 232: 58–65. [ Links ]
24. Ellis LM. A perspective on sentinel lymph node biopsy in colorectal cancer: The race between surgical technology and molecular oncology. Ann Surg Oncol 2000; 7: 475–6. [ Links ]
25. Cutait R, lves VA, Lopes LC, Cutait DE, Borges JL, Singer J, Da Silva JH, Goffi FS. Restaging of colorectal cancer based on the identification of lymph node micrometastases through immunoperoxidase staining of CEA and cytokeratins. Dis Colon Rectum 1991; 34: 917–20. [ Links ]
26. Jeffers MD, O'Dowd GM, Mulcahy H, Stagg M, O'Donoghue DP, Toner M. The prognostic significance of immunohisto–chemically detected lymph node micrometastases in colorectal carcinoma. J Pathol 1994; 172: 183–7. [ Links ]
27. Mamounas E, Wieand S, Wolmark N, Bear HD, Atkins JN, Song K, Jones J, Rockette H. Comparative efficacy of adjuvant chemotherapy in patients with Duke's B versus Duke's C colon cancer: Results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C–01, C–02, C–03, and C–04). J Clin Oncol 1999; 17: 1349–55. [ Links ]
28. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. Efficacy of adjuvant fluoruracil and folinic acid in B2 colon cancer. J Clin Oncol 1999; 17: 1356–63. [ Links ]














