Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de investigación clínica
versão On-line ISSN 2564-8896versão impressa ISSN 0034-8376
Rev. invest. clín. vol.56 no.1 Ciudad de México Fev. 2004
Rev Invest Clín 2004; Vol. 56(1):32-37
ARTÍCULO ORIGINAL
Pharmacokinetics of digoxin in children with congestive heart failure aggravated by other diseases
Farmacocinética de la digoxina en
niños con insuficiencia cardiaca congestiva
agravada por otras enfermedades
Janett Flores Pérez*
Ismael Lares Asseff*,**
Hugo Juárez Olguín*, ***
*Laboratorio de Farmacología, Instituto Nacional de Pediatría (INP), Mèxico. **Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional (CIIDIR-IPN) Unidad Durango. *** Departamento de Farmacología, Facultad de Medicina, Universidad Nacional Autónoma de Mèxico.
Jesus Bobadilla Chávez****
Servicio de Cardiología, INP.
Correspondece and reprint request:
Hugo Juárez Olguín
Laboratotio de Farmacología.
Instituto Nacional de Pediatría
Avenida Imán No. 1, 3er Piso Col. Cuicuilco,
04530 México, D,F,
Tel.& Fax: 10 8409-00 ext. 1426.
E-mail :juarezol@yahoo.com
Recibido el 10 de abril de 2003
Aceptado el 25 de septiembre de 2003
ABSTRACT
Objective. To determine individual digoxin level variations and the effect of some common diseases those aggravate congestive heart failure (CHF) on digoxin pharmacokinetics in children. Design. Digoxin pharmacokinetics was evaluated in 11 children with CHF and an additional disease, such as rheumatic fever, anemia or infections. Digoxin plasma levels were monitored in patients on multiple-dose regime. Setting. Third level pediatric hospital. Results. Pharmacokinetic parameters showed extensive variation; median values were: elimination half-life 42.0 hrs (8.3-77.0), volume of distribution 1.01 L/kg (0.654-6.25), and clearance 15.0 mL/kg/h (6.0-331.8), which differed from results in patients with only CHF, reported previously. Dosage schemes in use at the Cardiology Service produced the following results: 40.5% of patients reached therapeutic levels, 10.8% toxic levels and 48.6% sub-therapeutic levels. Conclusion. The range of dosage required in order to adjust individual treatments was very wide, leading us to the conclusion that therapeutic schemes for this population should be individualized based on their pharmacokinetic parameters, and therapeutic monitoring of drugs should be performed.
KEY WORDS. Children. Digoxin. Drug monitoring. Heart failu-re. Pharmacokinetics.
RESUMEN
El objetivo del presente estudio es determinar el efecto de algunas enfermedades que agravan la insuficiencia cardiaca congestiva (ICC) sobre la farmacocinètica de digoxina en niños. Diseño. Se estudió la farmacocinètica de digoxina en 11 niños con ICC, agravada por otras enfermedades como fiebre reumática, anemia o infecciones. Posteriormente se llevó a cabo monitoreo de los niveles de digoxina mientras seguían un règimen de dosis múltiple. Lugar. Centro de atención de tercer nivel. Resultados. Los parámetros farmacocinèticos mostraron amplia variabilidad: los valores de las medianas fueron: vida media de eliminación 42.0 horas (8.3-77.0), volumen de distribución 1.01 L/kg (0.654-6.25), y depuración 15.0 mL/kg/h (6.0-331.8) los cuales son diferentes a los de los pacientes que únicamente padecen ICC previamente reportados. Con el esquema de dosis usado en el Servicio de Cardiología se encontraron los siguientes resultados: 40.5% de las muestras de pacientes alcanzaron niveles terapèuticos, 10.8% niveles tóxicos y 48.6% subterapèuticos. Conclusión. Debido a la variación en los parámetros farmacocinèticos individuales, ocasionados por las patologías que agravan la ICC, se recomienda individualizar los esquemas de dosificación a partir de criterios farmacocinèticos, sin prescindir del monitoreo terapèutico del fármaco.
PALABRAS CLAVE. Digoxina. Farmacocinètica. Monitoreo terapèutico. Insuficiencia cardiaca. Pediatría.
INTRODUCTION
Most pharmacokinetic studies found in the literature have been performed in healthy adults. Some trials have been conducted in children, but very few have been completed in severely ill children who suffer from congestive heart failure (CHF) associated with other diseases that further undermine their health. 1,2 Studies in adults with renal failure under digoxin treatment showed an increase in digoxin serum levels and a prolonged half-life, which could lead to digitalis intoxication. 3 Several reports in the literature indicate that digoxin serum levels in infants and older children are higher than in adults. 4,5 Some authors recommend the use of small doses of digoxin in premature children, presumably due to reduced renal function compared with older children and adults. 6,7 As a result, digoxin dosage schemes have been based on trials performed in various populations, without considering the effect some disorders may have on the pharmacokinetics of this drug. This study was performed to describe the variations in the natural evolution of digoxin plasma concentration in pediatric patients with heart failure, independently from age and gender, and to determine the effect of certain disease conditions on digoxin pharmacokinetics in children, taking into account the drug pharmacokinetics during the disease process in patients treated with digoxin. Similarly, digoxin levels were monitored with the purpose of assessing plasma concentrations and performing a kinetic evaluation of the therapeutic scheme, to consider dosage adjustments based on individual pharmacokinetic parameters.
MATERIAL AND METHODS
The study included 11 children from the Emergency Ward at the Instituto Nacional de Pediatría (National Institute of Pediatrics), Mexico City, diagnosed with CHF secondary to diverse etiologies that required treatment with oral digitalis. In some cases, erythropheresis was required. The study was approved by the Ethics Committee, and an informed consent was obtained from the parents of children included in the study. The digoxin dose was estimated based on the recommended treatment scheme followed by the Cardiology Service of the Institute. A 0.5 mL blood sample was obtained at 0, 30, 60 and 90 minutes, and 3, 5, 10, 15, 24, 30, 35, 40 and 48 hours after digoxin administration. The study was performed in other wards different to the emergency room where the children were admitted due to their health condition; as a standard hospital procedure, children are not allowed to stay for more than three days in the emergency room. Digoxin plasma levels were monitored while patients received their multiple-dose regime, collecting blood samples before the administration of each maintenance dose. Digoxin plasma levels were measured with a radioimmunoassay technique and a gamma-ray counting device manufactured by Abbott. The method was validated running several calibration curves with increasing concentrations, based on the quality control system followed in the laboratory. The pharmacokinetic analysis was done using the Winnolin program version 1.1, 8 which considers the logarithms of plasma concentration-time and estimates linear regression slopes; in addition, it analyzes the behavior of the pharmacokinetic data and fits them to the best pharmacokinetic model with first order elimination kinetics. The predictive and simulation models used to measure steady-state maximum (C max ) and minimum (C min ) plasma concentrations were estimated with the Ritschel method. 9
RESULTS
Age ranged between 1 month, 10 days and 15 years, 11 months. Six male and five female patients were included with clear signs of CHF. In addition to CHF, the children had other diseases: 2 had rheumatic fever, 5 had anemia of diverse etiology, and 5 had lower respiratory tract infections. The remaining conditions are shown in (table 1).
(Table 2) shows the individual values of the pharmacokinetic parameters. Values vary considerably. For example, the elimination half-life (t ½ ) ranged from 8.3 to 77.0 hours with a median value of 42.0 hours; the distribution volume (Vd) ranged from 0.654 to 6.25 L/kg with a median value of 1.01 L/kg: total body clearance (Cl) ranged from 6.0 to 331.0 mL/kg/h, with a median of 15.0 mL/kg/h. The remaining pharmacokinetic parameters also showed important variations. Based on population heterogeneity, children were grouped according to age. Group I comprised children from 1 month to 3 years of age, and group II included children aged between 8 and 15 years. The analysis of the effect of age on drug distribution showed significant differences (p < 0.05): group II had a mean α value (distribution rate constant) of 0.2419 h -1 (range 0.0928- 0.7340 h -1 ), while group I had a mean α value of 0.8682 h - 1 (range 0.3472 - 1.1254 h -1 ). Similarly, distribution half-life (t ½ α) was longer in the second group, with a median of 2.86 h (range 0.937-7.46 h). Group I showed a median value of 0.814 h (range 0.6157-1.22 h); the remaining results are shown in (table 3). Grouping of children according to their drug-biotransformation ability disclosed two groups: slow eliminators, with an elimination half-life longer than 1.6 days and fast eliminators, with a shorter half-life. Significant statistical differences were found (p < 0.01) for several pharmacokinetic parameters, such as k 21 (transference constant), with a median value of 0.1451 (range 0.0470 - 0.2504 h -1 ) among slow eliminators, and 0.2944 (range 0.1656 - 0.8634 h-1) among fast eliminators. Similarly, the results for the area under curve from zero to infinity were higher among slow eliminators, with a median value of 213.56 (range 120.22 - 873.24 μg/mL/h), while fast eliminators showed a lower mean value of 41.25 (range 8.53- 171.8 μg/mL/h) (Table 4).
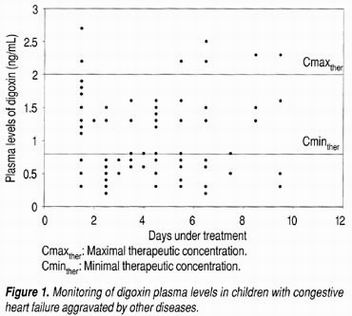
(Figure 1) shows the evolution of daily therapeutic digoxin monitoring during the treatment period. Extensive dispersion of the concentrations can be observed along the treatment period. A total of 74 samples were analyzed, corresponding to the minimum concentrations, of which only 40.5% reached therapeutic levels. Approximately 10.8% exceeded the therapeutic level with risk of toxicity, and 48.64% were sub-therapeutic.
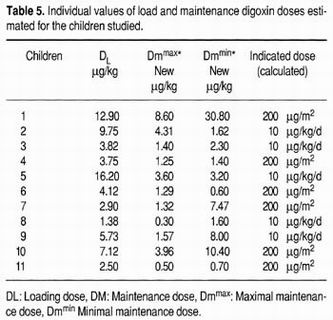
Dosage schemes were re-calculated for patients with digoxin concentrations above the recommended therapeutic range to reach a sustained therapeutic level. The loading and maintenance doses were estimated based on the results of the pharmacokinetic study for each patient, and are shown in (Table 5).
DISCUSSION
Disease severity confers special physiologic characteristics to the individual, which affect various organs or systems and modify the pharmacokinetic behavior of several drugs. 10,11 The assessment of digoxin plasma levels and the use of pharmacokinetic parameters are not common practice in our environment, and the authors consider it important to emphasize the implications and risks of neglecting this practice. The study group was interested in determining the natural development of digoxin plasma concentration among pediatric patients with heart failure treated with digoxin, independently of age, gender, or other factors, in order to demonstrate the importance of digoxin monitoring and of the pharmacokinetic differences that require individual management as a consequence of inter-individual variations observed in the present study and other reported investigations. Thus, the aim of this work was precisely to justify digoxin monitoring and to obtain individual pharmacokinetic parameters to optimize the rational use of digoxin, when future concentrations are unpredictable due to the presence of various factors. The variable and longer elimination half-lives observed in this study compared to results found in the literature definitely point to different populations, in view of the fact that findings reported elsewhere are derived from children with CHF, 10 while present results were obtained from severely ill children suffering from CHF aggravated by other diseases that complicated their health condition. In addition, children were classified as fast- or slow eliminators based on their ability to biotransform and/or excrete digoxin. This feature is determined by the effects of heart failure on renal and liver clearance, 12 resulting in altered distribution due to blood flow modifications. Similarly, heart failure has an effect on the distribution volume of certain drugs, which is usually decreased among these patients. 3 The effects of changes in the circulatory system on digoxin distribution to deep tissues is a result of hypoperfusion, often seen in patients with hypovolemic shock and/or heart failure. 13 Therapeutic failure is a consequence of inter-individual variability in the digoxin pharmacokinetic behavior, and is more common among severely ill patients suffering from hyperdynamic conditions resulting from the physiological implications related to their pathological condition. 2 Considering the wide variety of doses required as a result of this pharmacokinetic variability, it is important to establish individual treatment schemes and to conduct close therapeutic digoxin monitoring. Medical management control can be improved for these conditions, as suggested by several authors. 14,15 Once the evolution of plasma concentrations during a multiple-dose scheme has been determined, therapeutic failure can be established. Over 11% of the digoxin-concentrations monitored during treatment were found to be toxic, 49% were below therapeutic levels, large fluctuations between maximum and minimum concentrations were observed, and only 40% were within therapeutic range. Based on the results of the analyzed variables, it may be concluded that the reason for the decrease in recommended loading and maintenance doses in the study group was a result of the patients' different capacities to biotransform and/or excrete digoxin, reflected by the pharmacokinetic changes mainly on elimination half-life or distribution volume, since these parameters are fundamental for the establishment of adequate dosage regimes. So far, pediatric patients cannot be classified according to the severity of their cardiac failure in the same way as adults. This limiting factor obstructs the evaluation of effects of cardiac failure severity on digoxin pharmacokinetics. Additionally, a general conclusion is that strict drug monitoring of any critically ill or unstable patient treated with digoxin is required. Acknowledgements We thank Isabel Pèrez Montfort for correcting and editing this manuscript
REFERENCES
1. Steinberg C, Notterman DA. Pharmacokinetics drugs in children. Inotropes and vasopressors. Clin Pharmacokinetics 1994; 27: 345-67. [ Links ]
2. Steinberg C, Notterman DA. Pharmacokinetics of cardiovascular drugs in children. inotropes and vasopressors. Clin Pharmacokinet 1994; 27 :345-67. [ Links ]
3. Riaz K, Forker AD. Digoxin use in congestive heart failure. Drugs 1988; 55: 747-58. [ Links ]
4. Miura T, Kojima R. Sugiura Y, Mizutani M, Takatsu F, Suzuki Y. Effect of aging on the incidence of digoxin toxicity. Ann Pharmacother 2000; 34; 427-32. [ Links ]
5. Cambonie G, Haack K, Guyon G, Badr M, Fournier-Favre S, Souksi I, Guillaumont S. Digitalis intoxication during the neonatal period: role of dehydration. Arch Pediatr 2000; 7: 633-6. [ Links ]
6. Bendayan R, McKenzie MW. Digoxin pharmacokinetics and dosage requirements in pediatric patients. Clin Pharm 1983; 2: 224-35. [ Links ]
7. Sorkness R. Prolonged digoxin elimination in two patients with renal and hepatic disease. Clin Pharm 1983; 2: 271-3. [ Links ]
8. Winnolin program, Standard Edition, version 1.1, Scientific Consulting Inc. 1996. [ Links ]
9. Ritschel WA. Graphic approach to clinical pharmacokinetics. 2nd. ed. Barcelona: J. R. Prous. Publishers; 1984, p. 36-41. [ Links ]
10. Hauptman PJ, Kelly RA. Digitalis, cardiovascular drugs. Circulation 1999; 99; 1265-76. [ Links ]
11. Aronson JK. Clinical pharmacokinetics of cardiac glycosides in patients with renal dysfunction. Clin Pharmacokinet 1983; 8: 155-178. [ Links ]
12. Cheng JW, Charland SL, Shaw LM, Kobrin S, Goldfarb S, Stanek EJ. Is the volume of distribution of digoxin reduced in patients with renal disfunction? Determining digoxin pharmacokinetics by fluorescence polarization immunoassay. Pharmacotherapy 1997; 17: 584-90. [ Links ]
13. Kearney K. Emergency. Digitals Toxicity. Am J Nurs 2000; 100: 51-53. [ Links ]
14. Mooradian AD. Digitalis: An update of clinical pharmacokinetics, therapeutic monitoring techniques and treatment recommendations. Clin Pharmacokinet 1988; 15: 165-79. [ Links ]
15. Abad-Santos F, Carcas AJ, Ibanez C, Frias J. Digoxin level and clinical manifestations as determinants in the diagnosis of digoxin toxicity. Ther Drug Monit 2000; 22: 163-8. [ Links ]