Introduction
Neuroendocrine tumors are a group of neoplasms that arise from cells in the neuroendocrine system. Based on clinical and molecular data, two categories have been distinguished: typical or atypical neuroendocrine tumors, commonly called pulmonary carcinoid tumors (PCT); and high-grade neuroendocrine tumors (HGNET), which includes large cell and small cell carcinoma. PCT represents only 1-5% of all pulmonary neoplasms, with an estimated incidence of 5-10/1,000,000 people/year.1-9 The differentiation between typical carcinoids (TC) and atypical carcinoids (AC) depends on the count of mitotic cells (TC 0-2 and AC 2-10 by 2-mm2) and the presence of necrosis (AC). Compared to HGNET, PCT are less aggressive and generally follow an indolent course. ACs exhibit slightly more aggressive behavior with a higher recurrence rate and tendency to metastasize when compared to TC.2,6 Patients with TC and AC are typically younger than those with HGNET, without a predilection of sex, and do not appear to be related to smoking.3 PCT is often located centrally relative to the airway, predominantly intraluminal, although up to 30% are found in periphery locations and without invasion to adjacent tissues.2,4,10,11 They are usually solitary lesions (approximately 95% of cases).4 The most consistent histologic finding is cytological uniformity of the cell nucleus with little cell-to-cell variation. In addition, chromatin is described as patron of «salt and pepper», with a chromatin dotted without intranuclear vesicular chromatin or prominent nucleolus.3,4 Immunohistochemistry is a useful supplement for morphological evaluation. Cytokeratin immunoreactivity is present in TCP; however, up to 10% are negative for cytokeratin AE1:AE3. Neuroendocrine markers are often also performed, including synaptophysin, chromogranin and CD56. Because TCP is relatively well differentiated, these markers are relatively sensitive in this context, with chromogranin being the most specific.4 The use of Ki-67 could help in this type of tumor and has already been implemented in neuroendocrine tumors of other organs. In fact, in small biopsies, the use of Ki-67, especially in limited samples or samples with suboptimal morphology, can help distinguish low-grade neuroendocrine tumors from high-grade neuroendocrine tumors; that is, very low values (< 5%) versus high values (> 50%).4,5 Ki-67 is typically less than 5% Rin CT, less than 20% in CA and greater than or equal to 20% in TNEAG. If Ki-67 delivers additional value to histological evaluation in TCP, it remains an ongoing debate. However, Ki-67 may be useful in limited samples or crushed biopsies, where mitotic counting is virtually unworkable; Ki-67 less than 20% and greater than or equal to 20% favors TCP and TNEAG, respectively.6
Computed tomography (CT) is the imaging method of choice for the study of PCT patients. In centrally located tumors, diagnosis can be achieved by bronchoscopy and biopsy; while in peripheral lesions, transthoracic needle biopsy may be considered.1,7-9
Patients should be stationed in accordance with the 8th edition of TNM.10,11 Both conventional and positron emission images (PET-CT) can be used for staging.12,13 The N2 staging does not represent a contraindication for surgery, due to the indolent course of a significant number of patients. Many surgeons offer surgery for patients with clinical N2, provided the primary tumor is resectable.14-17 Imaging studies are also crucial for determining distant metastasis. The most common sites for PCT metastasis are the liver, bones, and mediastinal lymph nodes.14,18,19
In PCT surgery is the recommended treatment approach whenever possible, due to its low sensitivity to chemo-radiation therapy.16,18,20 Survival is generally favorable, with an estimated 5-year rate of 61-88% for AC and > 90% for TC (1-4). In TC, lobectomy or sublobar resection with free margins and the study of lymph nodes is recommended. In AC, lobectomy, or pneumonectomy with systematic lymphadenectomy should be performed.1-4,6,14,21 In advanced cases the prognosis is worse, with a survival rate of approximately five years of 27%, and without an existing standard treatment approach.1,22,23 Systemic therapy represents the main option in advanced, non-resectable disease; accepted alternatives are somatostatin analogues, peptide receptor radionuclides therapy, everolimus and chemotherapy. To date, an unequivocal strategy has not been identified, e.g. custom therapeutic algorithms should consider the efficacy of treatment as well as safety profiles.18,24-26. CA have a high incidence of recurrence, especially in N+.2,27 The histological subtype and the condition of the lymph nodes are the most important prognostic factors.1,28,29 Data on follow-up are controversial, long follow-up periods of up to 20 years1 are generally recommended. In patients with TC, recommended follow-up with CT at 3-6 months and then annually. For patients with CA, closer monitoring is recommended, first at 3-6 months and then at 6-month intervals.1-4,18 Complications are often reported, with an overall complication rate of surgery going from 0 to 30% depending on the different series.15,26 The most reported are prolonged air leakage, respiratory infections, cardiac arrhythmias, and secondary empyema.2,27,30
Material and methods
A retrospective search was carried of patients diagnosed with pulmonary carcinoid tumor, treated in March 2013-December 2019 within the National Institute of Respiratory Diseases. A cohort of 30 patients with tissue biopsy diagnostic of pulmonary carcinoid tumor was identified.
The variables studied included patient demographics, pathology report, clinical presentation, tumor location, type of surgery performed, complications, number of positive mediastinal nodes, adjuvance and follow-up performed. Patients who were not candidates for surgical management were excluded.
As it is a retrospective study, no variable was directly manipulated and it is considered a risk-free investigation, so the file review was carried out with authorization signed by the Ethics Committee of the National Institute of Respiratory Diseases and the head of the Department of Respiratory Diseases.
Results
30 patients diagnosed with carcinoid tumor were found. The median age was 47.2 years (range 17-73 years), with predominance of the female sex (66%). The average symptom evolution time was 28.4 months (range 2-69 months). In 50% of patients there was a history of smoking, and the most found comorbidities were high blood pressure (30%) diabetes mellitus (16%). The most common symptoms of presentation were progressive dyspnea (40%), hemoptoic (40%), cough with expectoration and fever (30%), weight loss (23%), and chest pain (20%) (Table 1).
Table 1: Description of patient population.
| Demograph feature | n (%) |
|---|---|
| Gender Female Male |
20 (66) 10 (33) |
| Age (average) (years) Hospital stay (media) (days) Smoking Arterial hypertension Diabetes mellitus |
47.2 20 15 (50) 9 (30) 4 (16) |
| Presentation symptoms*
Dyspnea Hemoptoic Cough with expectation Weight loss Thoracic pain |
12 (40) 12 (40) 9 (30) 7 (23) 6 (20) |
| Follow (media) (months) | 21.9 |
* Most patients had more than one symptom, the predominant one was considered.
The tumor appeared in 70% of cases on the right side and 30% on the left side; being the most common location in the lower lobe (30%), followed by the main bronchi, upper and middle lobe (20% for each). All patients had a contrasted CT study as a preoperative protocol (Figures 1 to 3). Diagnosis of carcinoid tumor was made preoperatively through biopsy during bronchoscopy in 90% of cases, in the rest it was postoperative (Table 2).

Figure 1: Representative section of contrasted computed tomography in the pulmonary phase at the level of the pulmonary arteries, where an endobronchial tumor is observed at the level of the intermediate bronchus (arrowhead), homogeneous, without contrast medium enhancement, occluding almost 100% of the bronchial lumen.

Figure 2: Representative section of contrasted computed tomography in the mediastinal phase, where an endobronchial tumor is observed at the level of the bronchus of the lower lobe (arrowhead), heterogeneous, with a slight enhancement to the contrast medium.
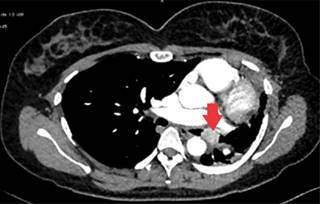
Figure 3: Representative contrast computed tomography section in the mediastinal phase showing an endobronchial tumor at the level of the left main bronchus (arrowhead), which conditions atelectasis of the left lung and deviation of the ipsilateral mediastinum, homogeneous and with enhancement to the contrast medium.
Table 2: Anatomical and histopatological description.
| Location | n (%) |
|---|---|
| Right Upper lobe Middle lobe Lower lobe Main bronchus Superimposed in 2 lobes |
21 (70) 6 (20) 6 (20) 3 (10) 3 (10) 3 (10) |
| Left Upper lobe Lower lobe Main bronchus |
9 (30) 0 6 (20) 3 (10) |
| Histopathology Typical carcinoid Atypical carcinoid |
18 (60) 12 (40) |
| Tumoral size < 2 cm 2-4 cm < 4 cm |
7 (23) 13 (43) 10 (33) |
| Ganglionar involvement No Yes No nodes examined |
22 (73) 4 (13) 4 (13) |
The surgical approach was by thoracotomy (63%), followed by conversion from VATS to thoracotomy (20%) VATS (16%). The surgical procedure performed was lobectomy in 66% of cases, followed by 20% of pneumonectomies, lobectomy with bronchoplasty in 16% of cases. 6% bilobectomy and 6% sleeve resection (Table 3).
Table 3: Surgical description.
| Approach | n (%) |
|---|---|
| Thoracotomy VATS VATS with conversion to thoracotomy |
19 (63) 5 (16) 6 (20) |
| Procedure Lobectomy Pneumonectomy Bilobectomy Sleeve resection Lobectomy + bronchoplasty |
15 (50) 6 (20) 2 (6) 2 (6) 5 (16) |
| Complications Bleeding Pneumonia Fistula broncopleural |
7 (30) 4 (13) 2 (6) 1 (3) |
| Adyuvance Mortality at 30 days |
4 (13) 0 (0) |
VATS = Video-assisted thoracoscopic surgery.
The number of stations dissected during lymphadenectomy was varied (between 1 and 6 stations), and in four cases it was not performed. In 4 cases (13%) there were positive ganglion metastases, two cases at station 7 and two at station 4. The average tumor size was 3.21 cm (range 0.5-10 cm) (Table 2).
In 7 cases (23%) complications were present, the most common was transoperative bleeding greater than 600 mL that required blood transfusion in 4 cases (13%), followed by hospital pneumonia in two cases (6%); and one patient (3%) post-pneumonectomy bronchopleural fistula development. The overall hospital stay averaged 20 days (range 5-47 days), in 4 cases (13%) there was a need for stay in Intensive Therapy, two for mass transfusion and two for hospital pneumonia (Table 3).
In pathological results, 60% of cases were a typical carcinoid and 40% an atypical carcinoid (Figures 4 to 6). In 23 cases (76%) surgical resection was R0, in 4 cases (13%) was R1, in one case (3%) R2 and in two cases the surgical margin was not specified in the pathology report. Four patients (13%) received adjuvance with chemotherapy, and 3 of them radiation therapy; in two cases these were patients with positive margins and atypical carcinoid; another had an R2 surgical resection, and another did not exhibit any prominent factors. None of the patients with positive ganglion metastases received adjuvance. In two cases with positive surgical margins, only surveillance was given (Table 2).

Figure 4: Photomicrograph showing the characteristic appearance of a typical carcinoid tumor composed of cells with moderate to scant cytoplasm, slightly clear, nuclei of fine chromatin in salt and pepper, without atypical pleomorphism or mitoses. Photo courtesy of the INER anatomopathological service. Dr. Luna.

Figure 5: Photomicrograph showing the characteristic histological appearance of an atypical carcinoid tumor with a solid growth pattern and formed by abundant cells. The cells are medium to large, round, and polygonal with abundant cytoplasm, round nuclei and grouped in an organoid pattern. There is no necrosis and mitosis 3 to 4 in 2-mm2. Photo courtesy of the INER anatomopathological service. Dr. Luna.
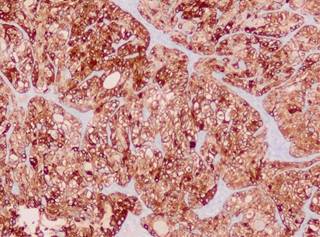
Figure 6: Positive CD56/NCAM immunohistochemistry demonstrating the neuroendocrine nature of the tumor. Photo courtesy of the INER anatomopathological service. Dr. Luna.
Most of the patients were found in early stages, according to TNM (Tumor, Nodes, Metastasis) 8th edition guidelines,31 the most frequent being stages IA2, IA3 and IB (Table 4). Long-term follow-up averaged 21.9 months (2-60-month range) between the years of 2015 and 2019, 100% of patients were alive without recurrence at their last revision. However, there was a 36% (11 cases) patient loss, who no longer attended successive appointments. There was no mortality reported after 30 days.
Table 4: Staging according to TNM 8th edition.31
| Estadio | n (%) |
|---|---|
| IA1 | 1 (3) |
| IA2 | 7 (23) |
| IA3 | 6 (20) |
| IB | 5 (16) |
| IIA | 3 (10) |
| IIB | 3 (10) |
| IIIA | 4 (13) |
| IIIB | 1 (3) |
| IIIC | 0 (0) |
| IVA | 0 (0) |
| IVB | 0 (0) |
TNM= Tumor, nodes, metastasis.
Discussion
This retrospective study shows the cases of carcinoid tumor reported in our institute over a period of 5 years. The demographic data are comparable to that reported in the literature, with a female predominance of 2: 1; a mean age of 47.2 years, highlighting the long period of time that patients can present symptoms, mainly dyspnea and haemoptoic sputum (from two months to five years). This is because they can easily be confused with other pathologies. Although 50% of the patients had positive smoking, it is not considered to have a significant association with the development of the pathology. Regarding comorbidities, arterial hypertension and diabetes mellitus were the most found. The most widely used study in our environment to establish the diagnosis is bronchoscopy, which reached a diagnostic yield in 90% of the cases. Regarding the anatomical location, a predominance of the right side stood out with 70%, without a clear predominance among any of the lobes. In our series, posterolateral thoracotomy was used as a surgical approach in a greater number of cases; However, in recent years the use of VATS as the one of choice has increased, as experience with this approach has improved within our Institute. The type of procedure performed was very varied, which shows that it is a pathology that requires extensive knowledge of surgical techniques in the thoracic surgeon. The main procedure being lobectomy in 50% of cases, followed by pneumonectomy and lobectomy with bronchoplasty in 20 and 16% of cases, respectively. In most cases it was accompanied by selective lymphadenectomy. The main complication was significant intraoperative bleeding that required blood transfusion; however, only one of these patients merited packaging and reoperation for this reason. The patient who developed a bronchopleural fistula in the postoperative follow-up, required endoscopic treatment for its resolution. Regarding the pathological result, there was a predominance of cases of typical carcinoid tumor in 60% and of atypical carcinoid in 40%. Tumor size ranged from 0.5 cm to 10 cm, with a predominance between 2 and 4 cm in 43% of cases. Most of the cases were early stages, mainly stages IA2, IA3 and IB. In most cases an R0 surgical resection was achieved, which is always desired in this type of patient. The disparity in the adjuvant treatment used in patients is striking, which is a work area for our Institute in creating established protocols for this type of patients. There was no mortality after 30 days in any operated patient. Finally, no recurrence was found in any patient during follow-up; however, given the slow growth of this type of tumors, the follow-up time may be short to detect recurrence, so it should be given for much longer as recommended by the literature.
Conclusions
Pulmonary carcinoid tumors are relatively rare pulmonary neoplasms that occur with nonspecific and long-evolving symptoms. They can occur at different levels within the bronchial tree or in any lobe, without a established predominance. Diagnosis is usually achieved preoperatively by bronchoscopy; however, surgical resection is required for treatment, and where feasible, with as much preservation of lung tissue as possible. The procedure of choice is lobectomy with lymphadenectomy, although in these patients’ other types of procedures may be necessary, such as bronchoplasty or sleeve resection, in order to achieve a complete R0 resection. This leads to excellent local control and high long-term survival. Atypical histology and the involving of lymph nodes are recognized as the most important adverse prognostic factors. More randomized studies are required to establish optimal management for patients with primary lung carcinoid tumors.











 text new page (beta)
text new page (beta)


