Introduction
The chemical composition of the groundwater results from the interaction of rainwater with the dissolved rocks in the recharge zone (where it acquires part of its components). After filtration, it continues acquiring ions by its contact with the granular material through which it moves. The carbonate rocks undergo a process of chemical alteration by the action of rainwater that is combined with carbonic acid, this acid water dissolves the carbonate rocks, giving rise to a karstic landscape. In karst areas, the groundwater flow is characterized by high speeds, short residence times and significant water-rock interaction (Roback et al., 2001). When the water infiltrates by small fractures, the solution goes deepening and widening these fissures, creating a network of ducts in the karstic rock. This process increases its secondary permeability and induces the formation of karst (Kiraly et al., 1979; Andreo et al., 2004; Antigüedad et al., 2008). In their evolution, groundwater may interact with other currents or groundwater flows, resulting in mixing of waters of different chemical composition due to natural or anthropogenic processes (Harpaz and Bear, 1963; Ramos et al., 2007). A way of analyzing this phenomenon in generating isovalue curves of the different ions contained in the water, which allows to crossing directions of flow and zones where they can occur encounters of subterranean currents with different chemical compositions. Another way to organize the contributions of water mixtures in the groundwater flow is to use the conservative elements, frequently considered as tracers. The blends may be mainly binary or ternary; simultaneous mixtures with more than three end-members are uncommon to occur in nature whereas binary mixtures are very common. For example, to quantify the mixture of fresh and marine water have been widely explained in numerous publications (Apello and Postma, 1996; Generaux et al., 2004; Wallick, 1981; Lee and Krothe, 2001; Abu-Jaber, 2001; Skalbeck et al., 2002; Valentino and Stanzione, 2002). However, ternary mix models are less common. Rice and Hornberger (1998) evaluated the maximum flow contribution for groundwater in the US, and Generaux et al., (2004) evaluated groundwater mixtures by basin-to-basin transfer in the Costa Rica. Laaksoharju et al., (1999), have addressed simultaneous mixtures with more than three end-members (EM). However, these authors did not explain how this process is carried out in nature. In addition, Douglas et al., (2000) have reported that the mixing of three types of water with different chemical conditions and documented that this process can promote chemical reactions in an aquifer. In this work, we report that how chemical composition of water circulates through the volcanic rocks, limestone and alluvial fill in three different sub-basins. Therefore, the physicochemical characteristics, groundwater types, ion exchange processes and water-rock interaction of three different aquifers (San Nicolas Tolentino, Ocampo-Paraiso, Santa Catarina) are studied, which situated in the Zona Media of the state of San Luis Potosi, central Mexico. These findings contribute significantly due to their necessity in supplying drinking water as well as agricultural purposes. In addition, it provides valuable geochemical information for hydrogeology at local as well as a regional level.
Hydreogeological framework
The San Nicolás Tolentino aquifer (SNTA) located in the southern part of the state of San Luis Potosí (Figure 1) and it covers an exposed area of approximately 1,712.99 Km2. In this region, the climate is dry, arid with an average annual rainfall of 300 mm (INEGI, 2002) and an average temperature of 25° C (INEGI, 2002).
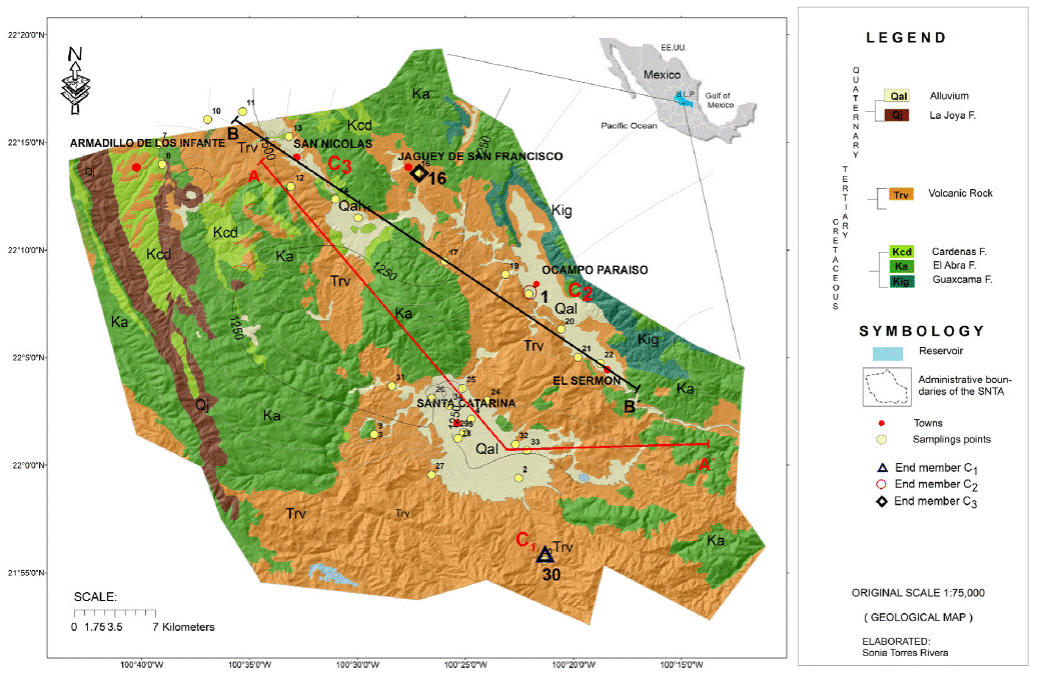
Figure 1 Location of the San Nicolas Tolentino Aquifer, regional geology of the study area and sampling points.
The SNTA consists of three sub-basins, drained by the Santa Catarina (sub-basin I), Ocampo Paraíso (sub-basin II) and San Nicolás Tolentino (sub-basin III) River (Figure 1).
The Santa Catarina sub-basin (I), is an exorheic sub-basin with an area of 732.5 km2, it is drained by the Santa Catarina River, which corresponds to 84.5% of the total area of the SNTA. Ocampo-Paraíso sub-basin (II) forms a sub-basin of 345.36 km2. San Nicolás sub-basin (III) is an endorheic sub-basin of the area of 369.62 km2 in which the Las Golondrinas dam is located.
Geology
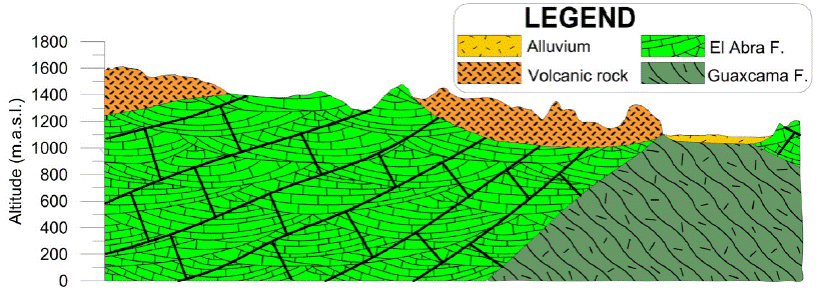
The oldest stratigraphic unit that outcrop in the region is known as Guaxcama Formation of Lower Cretaceous (Neocomian-Aptian), which is composed of anhydrite, gypsum, and dolomite (Carrillo, 1971). This formation developed large diapiric structures (Carrillo-Bravo, 1971; Torres-Hernández, 1994), and is covered by the thick more than 1000m calcareous sequence of the Abra Formation. The youngest Cretaceous unit is the Cardenas Formation, which is constituted by a sequence of shales and sandstones. Its thickness is irregular, between 50 and 100m. The central training unit to consider in this work is the Abra Formation, it is construed by lagunar and reef facies. This unit is widely distributed, and carved for the most significant karst development. It thicknesses is up to 1,800 m (Zapata-Zapata and Pérez-Venzor, 1979).
The Sierra de Alvarez (west of the studied area), constitutes the highest elevation in the region, and exposed as the slope foot facies (pre-reef deposits with 120 m of thickness), which is known as Tamabra Formation (López-Doncel, 2003).
The Cretaceous rocks are covered discordantly by volcanic rock sequences of andesitic and rhyolitic composition (Casa Blanca andesite, Santa María Ignimbrite, Portezuelo Latite, San Martin Avalanche, San Nicolás Formation, and Jagüey Formation; Torres Hernández et al., 2010). This set of Paleogene (Oligocene) volcanic units is mainly distributed in the south-central part of the study area, with thicknesses greater than 140 m (Labarthe-Hernández et al., 1982). Alluvium sediments cover all the units locally. Figure 2 shows a longitudinal geological section of the Peotillos, San Nicolás Tolentino and Santa Catarina valleys, looking towards the east (Figure 2). It can be observed that the Sierra de San Nicolás Tolentino is a remnant that belongs to a flank of an anticlinal that was eroded in Ocampo Paraiso sub-basin.
Hydrogeology
In the study area, aquifers are housed in granular material that partially covers Paleogene volcanic rocks, and carbonated rocks of the Cretaceous. Regionally, the largest aquifers are housed in limestone rocks.
The distribution of granular material is limited in the valleys, where it reaches only a few tens of meters, so its potential is not essential. The fracture medium represented by El Abra Formation that is the most crucial aquifer unit in the region because of its wide distribution and high thickness (Zapata and Pérez, 1979). This unit is exposed overall the high mountain, where it functions as a vital recharge zone, and in general, the flow goes from NW to SE, W to E, and in the southern part, of S to N (Figure 2). Since the origin of this unit belongs to the marine-platform and cover with reef and lagoon facies that provides it primary porosity whereas tectonic processes responsible for secondary porosity. In this región, El Abra Formation is very fractured, especially in the anticline nuclei where it develops numerous karstic structures (from sinkholes and uvalas to poljes). The processes of dissolution form cavities in the sub-soil, developing karstic caverns such as those of La Puente, El Ángel and La Catedral. Volcanic units with less distribution and thickness than limestones also function as aquifer units (Figure 2).
Methodology
Sampling and analysis
We collected a total of 34 water samples (26 correspond to wells, 8 to dugwells) whose distribution is shown in Figure 1, based on the "Groundwater Sample Protocol" (Armienta et al., 1987; Deutsch, 1997). During sample collection, physical-chemical parameters were measured in an isolation cell, which has limited interaction with the atmosphere, preventing changes in its original composition (temperature, electrical conductivity, pH, dissolved oxygen, oxide reduction potential and alkalinity). All groundwater samples were collected in linear high-density polyethylene (HDLP) bottles for the analysis of more abundant ions. The bottles were previously washed three times, with plenty of deionized water. The sample was acidified right after collection to pH <2 with ultrapure nitric acid. The samples for the determination of anions were not acidified. The major ions were analyzed in the Laboratories of Chemical Analysis, Faculty of Engineering, Autonomous University of San Luis Potosi. The alkalinity was performed in situ by the titration method of Gran. For the analysis of Ca2+, Mg2+, Na+ and K+, the Atomic Flame Absorption Spectrometry (FAA) method was used. The chlorides were analyzed by the Argentometric method, the Sulphates, and Fluor with the Turbidimetric method. All chemical analyses show an ionic balance less than close to 5% allowed (Freese and Cherry, 1979).
Calculation of Mixtures
The end-members represent the highest and lowest concentrations of the system. The mixtures types can be binary and ternary; the former has been used to quantify mixtures of freshwater and marine waters (Apello and Postman, 1996; Genereux et al., 2002; Wallick, 1981; Abu-Jaber, 2001, Skalbeck et al., 2002; Valentino and Stanzione 2002). (Laaksoharju et al., 1999; Douglas et al., 2000) showed multidimensional models with more than three water mixtures. As part of the methodology for the qualitative and quantitative analysis of a binary or ternary mixture, three end-members representing the evolution of the aquifer under study are identified.
The percentage calculation for each of the sub-basins was obtained using three equations with three unknowns; it is represented as a balanced equation:
Where C1 is the member associated with the local recharge in Santa Catarina, C2 is the member associated with Ocampo-Paraiso, and C3 is associated with the flows of San Nicolás Tolentino. C1, C2, and C3 are unknown values, and Cw is equal to 1.
These components were determined with the equations of the mass balance of two conservative elements Li+ and Br-:
(Equation 4) is obtained by substituting in Equation 2 in C3 of Equation 1:
C1 substitute for Equation 3 in Equation 1 and obtain the Equation 5:
The only possible solution for C2 is when Cw = 1. The other two components are calculated by clearing C2 and substituting C1 and C3 in Equation 1 and the Equation is obtained:
Results and discussion
The statistics of the chemical results of the groundwater samples are presented in Table 1. The results show that sub-basin I has a lower temperature, is rich in K+, Ca2+, HCO3 -, Cl-. Sub-basin II has an average temperature, lower ph, DO, Eh, Na+, K+, Ca2+ and higher Mg2+. The sub-basin III presents higher T, pH, EC, DO, Eh, Na+, SO4 2- and lower Alkalinity, hardness, Cl- .
Table 1 Statistics of the chemical results of sub-terrestrial water samples.
| Sub-basin | T | pH | EC | DO | Eh | TDS | Hard Total | Major Elements (mg/L) | ||||||
| °C | µS/cm | % | mV | mg/L | mg/L | Na+ | K+ | Ca2 | Mg2 | HCO3 - | Cl- | SO4 2- | ||
| I) SC | ||||||||||||||
| MIN | 24 | 7 | 309 | 2 | 234 | 200 | 61 | 24 | 6 | 16 | 2 | 134 | 7 | 14 |
| MAX | 31 | 9 | 1848 | 40 | 1816 | 1290 | 836 | 149 | 9 | 255 | 48 | 401 | 12 | 725 |
| MEAN | 27 | 8 | 610 | 18 | 516 | 419 | 210 | 51 | 6 | 62 | 13 | 217 | 9 | 118 |
| STD | 3 | 1 | 557 | 13 | 575 | 391 | 278 | 44 | 1 | 86 | 17 | 93 | 2 | 268 |
| II) OP | ||||||||||||||
| MIN | 21 | 8 | 449 | 2 | 81 | 320 | 148 | 36 | 4 | 41 | 232 | 232 | 7 | 29 |
| MAX | 34 | 9 | 810 | 24 | 340 | 600 | 336 | 54 | 8 | 83 | 366 | 366 | 30 | 115 |
| MEAN | 24.6 | 7.8 | 566 | 7.4 | 282 | 422 | 221 | 41 | 6 | 58 | 280 | 280 | 12 | 48 |
| STD | 4 | 0.4 | 118 | 7.4 | 80 | 96 | 61 | 6 | 1 | 12 | 46 | 46 | 7 | 29 |
| III) SNT | ||||||||||||||
| MIN | 16 | 8 | 1 | 6 | 275 | 400 | 205 | 27 | 4.1 | 59 | 267 | 267 | 11 | 2 |
| MAX | 26 | 8 | 808 | 20 | 344 | 600 | 301 | 77 | 25 | 90.1 | 390 | 390 | 35 | 53 |
| MEAN | 21 | 8 | 545 | 10 | 318 | 447 | 247 | 44 | 9 | 77 | 322 | 322 | 18 | 32 |
| STD | 3.1 | 0.2 | 208.6 | 4.4 | 20.6 | 66.0 | 29.3 | 15.4 | 5.9 | 10.1 | 35.7 | 35.7 | 8.3 | 16.1 |
I) Santa Catarina sub-basin (SC); II) Ocampo Paraiso sub-basin (OP) and III) San Nicolas Tolentino sub-basin (SNT)
Hydrogeochemical characterization
The chemical composition of groundwater is the result of continuous processes of interaction between precipitation water that infiltrates the subsoil and circulates through geological materials. Part of the chemical components is acquired in the recharge zone. Others are acquired along the pathway, captured by wells and in the discharge area as springs, resulting in different hydrogeochemical signatures.
Types of water
The Piper diagram (Piper, 1994) represents the end-members of each region and the classification of the different types of water (Figure 3). In the left part of the diamond, it displays mainly calcium-bicarbonate water. The upper part indicates dissolution of rock and middle part represents mixed waters where ion exchange can occur. On the left side of the diamond, is possibly the most evolved water, with dominance of the carbonate and sulfate ions.
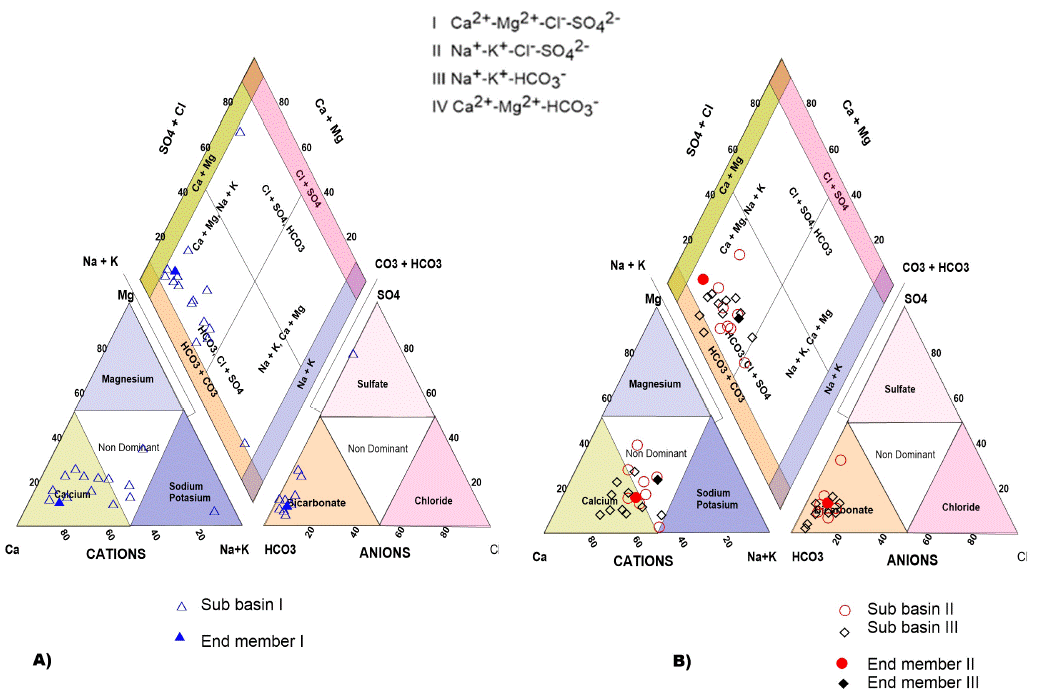
Figure 3 Piper diagrams: A) sub-basin I, Santa Catarina. B) sub-basins II and III Ocampo Paraiso and San Nicolas Tolentino for groundwater.
In general, three families were obtained for the study area: calcium bicarbonate (Ca-HCO3), sodium bicarbonate (Na-HCO3), and only one exotic sample that is classified as calcium sulfated (Ca-SO4) (Figures 3a and 3b).
In Santa Catarina sub-basin I, two aquifers hav e been detected (Torres-Rivera, 2012) that is known as free and confined, respectively. The water recharge area of the free aquifer is influenced by the Santa Catarina and San Martín rivers. The predominant water family is Ca-Na-HCO3 and Ca-HCO3 (Figure 3 A). These water families reflect that the most important source corresponds to the Santa Catarina River. Their main salt content corresponds to the interaction of the water with calcareous Ca-HCO3 family and movement of the water in the granular material, which consists predominantly products of the San Martín Avalanche (Torres-Hernández et al., 2010; Torres-Rivera, 2012). The mixing of the Ca-HCO3 water type with volcanic rocks interaction generates Ca-Na-HCO3 family. A single sample corresponds to Sulfate-Bicarbonate-Calcium-Magnesium composition, and additionally, it has 1290 mg/L of TDS; reflecting above all a much higher salt enrichment than all the other samples analyzed in this valley. This high value is considered as evidence that it reflects a regional flow and corresponds to a confined aquifer (Torres-Rivera, 2012). It also represents a confined aquifer, since the well that captures these waters penetrated beneath a thick ignimbrite layer (Santa María Ignimbrite), which functions as a seal rock of the same.
The waters family in the sub-basin II of Ocampo-Paraíso, is Ca-Na-HCO3, reflecting above all the interaction of the currents with calcareous rocks and the incorporation of the sodium liberated from the volcanic clasts in the granular medium.
In sub-basin III of San Nicolás, the Ca-Na-HCO3 and Ca-Na-HCO3-SO4 families were documented at the south of the Morenos town. This type of water reflects the interaction of the water with limestone rocks, gypsum, and ion exchange in the valleys where there is the presence of clay materials.
Hydrogeochemical processes with Gibbs diagrams
To identify some hydrogeochemical processes that occur in the groundwater, diagrams are used such as Gibbs diagrams (Gibbs, 1970), which was developed for surface water studies; however, in recent decades it has been used for the study of groundwater. The three main mechanisms that control the chemistry of surface water can be defined as the domain of atmospheric precipitation (rain), the domain of the rock, and domain of the evaporation-crystallization process (Gibbs, 1970). The Gibbs diagram was elaborated with the chemical data (anions and cations) of the groundwater samples (Figure 4).
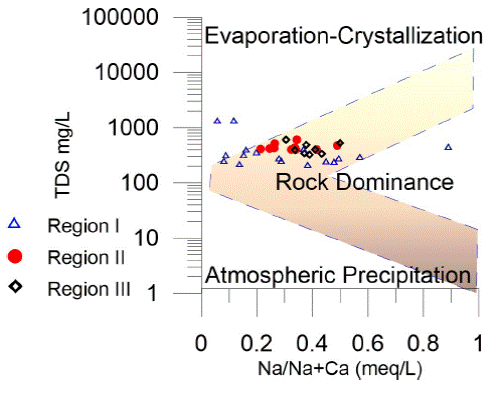
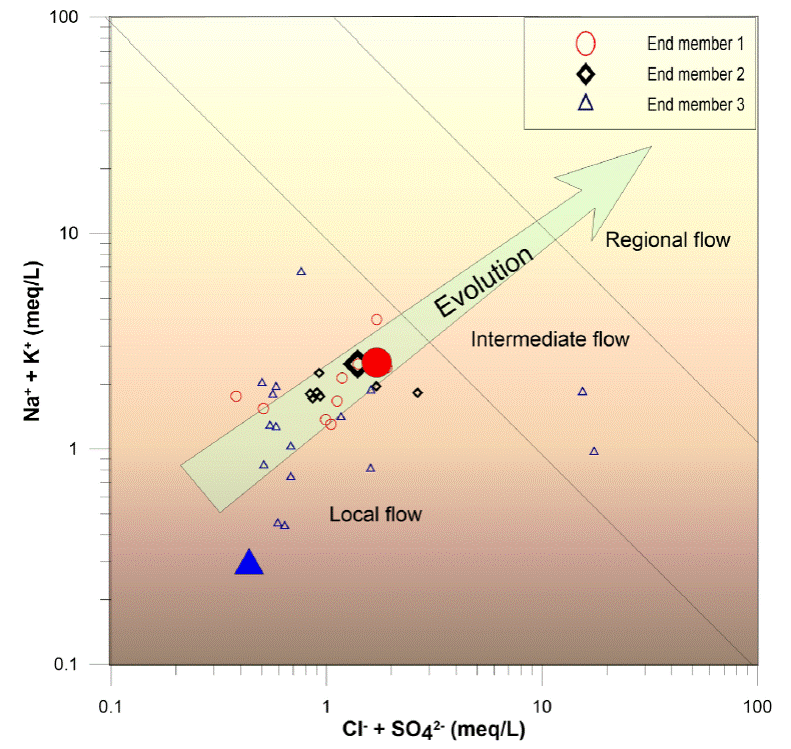
Figure 4 Gibbs diagram, the main evolutionary process that we identify with this diagram is the interaction rock water; the yellow arrow indicates the direction of the evolution of groundwater.
Based on Figure 4, we can infer that during its evolution, the water was influenced mainly by the water-rock interaction and a little evaporation due to the evaporation seems to have been dominant only in two samples. When the water infiltrates the subsoil, the water-rock interaction begins, the water from the recent infiltration has fewer chemical components; while the greater distance traveled, the residence time, the more significant the physical and chemical components increase; this evolutionary behavior, can be seen in Figure 4. Member I is very close to the vertical axis, which is associated with the water in the recharge zone while member II is away from the vertical axis because of its significant evolution.
Flow Systems
The increase of chemical components during the evolution of the groundwater can be observed in the Mifflin diagram (Mifflin, 1968) and uses hydrogeochemical indicators (Na+ + K+ + vs. Cl- + SO4 2-) to understand the evolution of groundwater and establish the different types of water flows (Figure 5). It is divided into three zones, and the area closest to the origin is due to the low circulation and short residence time of the water, the samples in this area are associated with the recharge (local flow). In the next zone, the concentrations increase and may be associated with a long-distance or longer residence time (intermediate flow).
Finally, the higher concentration is associated with more significant water-rock interaction and higher evolution of the groundwater (regional flow). In the study area, there are two types of water flow, one local and the other intermediate (Figure 5).
Hydrogeochemical Section
During its pathway, the groundwater interacts with the different materials, which results in the increase of the chemical components and in some cases, the decrease of these, due to the mixture with water of recent infiltration. To corroborate the water-rock interaction and mixtures a hydrogeological-hydrogeochemical, this section was elaborated.
The hydrogeochemical section is 40.5 km, begins in the Armadillo de los Infante, where volcanic rocks contact with the limestones and known as El Abra Formation, which cross the Ocampo valley of volcanic rocks and finally, cuts a valley where gypsum from the Guaxcamá Formation are found a few meters deep (Figure 6). (This section is only justified for the valleys of San Nicolas Tolentino and Ocampo Paraiso).
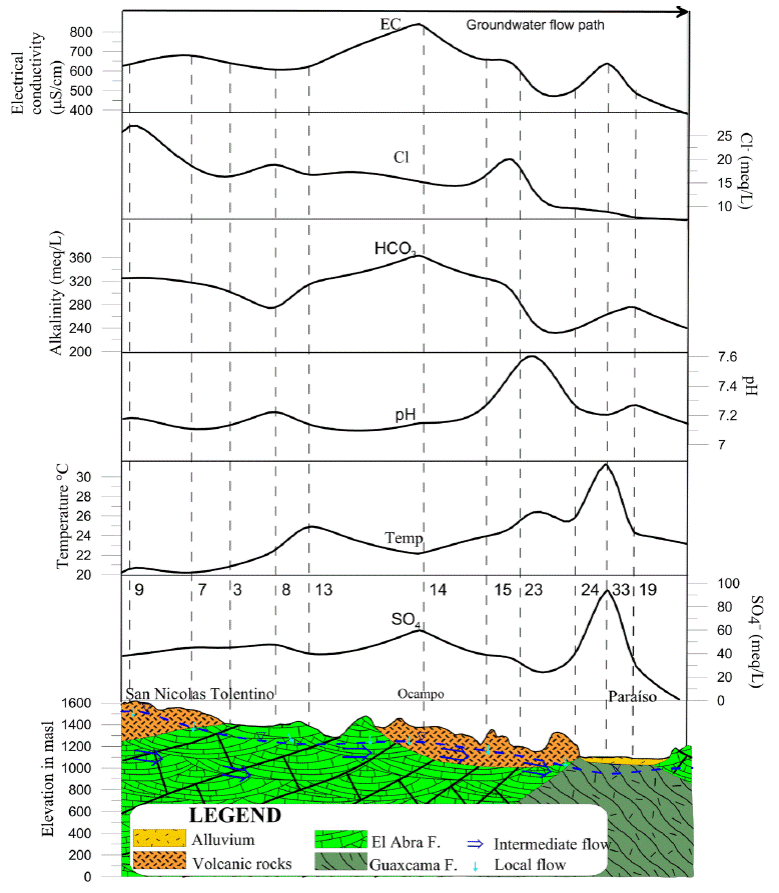
Figure 6 Hydrogeochemical section of Armadillo de los Infante to Paraiso; the numbers indicate several use; the discontinuous blue line indicates the piezometric level.
For the first sub-basin, the Ca-Na-HCO3 waters reflect the primary interaction with calcareous rocks of the El Abra Formation that crops out in the Sierra de Álvarez, which are partially dolomitized and contain varying amounts of magnesium in their dominant composition of Ca-HCO3. Ca-HCO3 its transit through the granular material that fills the Graben de Peotillos (López-Doncel et al., 2001) could print its sodium content indicates that this filling was deposited as a lacustrine fluvial material, which is due to having a shallow water tie. The location of the valley in an environment with medium to intense evaporation could include to some extent sodium. Another possible source of this sodium would be the decomposition of sodium plagioclase in the ignimbrite rocks, which interacts with the water currents and mix into the San Nicolas Tolentino river.
To the south of the Morenos town, where the San Nicolás River breaks its trajectory. As mentioned above, the first family waters (Ca-HCO3) acquire a character of Bicarbonate-Sulphated, perhaps due to the nearby influence of the gypsum that emerges in El Potrero anticline.
The physical parameters of sub-basins II and III, in the Armadillo de los Infante reveal that the electrical conductivity in the waters is approximately 650 µS/cm, along the hydrogeochemical section. The highest point is located at the center of the section in Paraiso, due to the interaction of the groundwater with the limestones whereas lowest point at the end of the section in Ocampo Paraiso town, because there is a mixture of local recharge water through the volcanic rocks. The concentration of chlorides is high at the beginning of the section, with values of 25 mg/L due to the agricultural activity that takes place in that area. Concentrations tend to decrease toward Ocampo Paraíso where it is possible for groundwater to mix with waters of recent infiltration. The alkalinity starts with values of 320 mg/L in Armadillo de los Infante and increases in the Ocampo zone due to the interaction of the water with the limestones, decreases to 240 mg/L at the end of the section due to mixing with water from recent infiltration (Figure 7).
The pH is 7.2 at the beginning and maintains little variation until Ocampo. However, it increases up to values of 7.6 and end with values of 7.2. The temperature starts at 20 °C and increases throughout the section until reaching 31 °C towards the end of the section in Paraiso. Sulfates begin with values close to 40 mg/L, increase slightly towards Ocampo town (60 mg/L) and on the Paraiso town increase to reach 100 mg/L; this increase can be related to the interaction of water with gypsums, which generates an exothermic reaction and this is congruent with the temperature anomaly in Paraiso.
Origin of the end-members and mixtures
To evaluate the contributions to a hydrogeological system, the mixture models are used, for which it is necessary to identify the end-members representative of each region.
Based on the scatter plot of the conditional elements i.e., Li and Br, which do not react chemically with the environment during the evolution of groundwater are used in the present study. The study represents three end members (C1, C2, C3) and corresponds to a ternary mixing. Two members are the most evolved and corresponds to intermediate flows while third one is associated with local recharge.
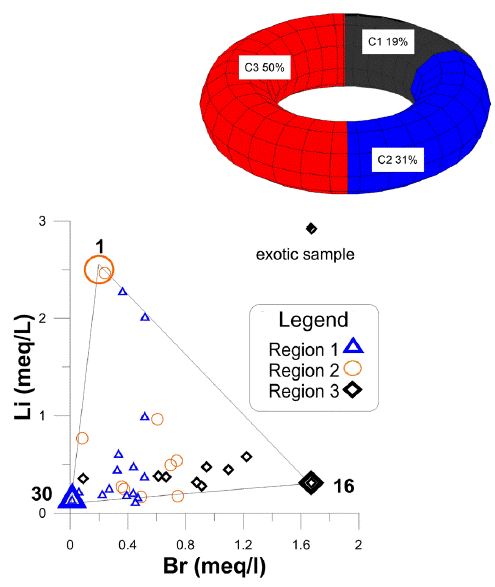
In Figure 7, a ternary mixing is identified, which is formed by (C1) associated with sub-basin I (Santa Catarina) for local recharge, (C2) associated with sub-basin II (Ocampo-Paraíso) and (C3) associated with the flow of sub-basin III (San Nicolás Tolentino). This figure display a triangle, in whose vertices the end-members are located. The remaining samples are between the limits of the mixture lines and are considered as a mixture of the fractions of the end member. Any water sample from the system can be generated utilizing the three end-members.
Sub-basin I, is represented by sample 30, which located to the south of the study area (Santa Catarina), towards the Sierra de Álvarez (Figure 1) and associated with local recharge and content low values of Cl-, SO4 2-, Br-, and Li+. In this sample the lowest values were measured for Cl- (0.22 meq/L), Li+ (0.128 meq/L) and 0.012 meq/L of Br- (Figure 7); the measured temperature was of 14 °C, with a pH of 6.5. The type of this member is Ca-HCO3.
Sub-basin II, sample 1, which corresponds to an intermediate flow, is located at the eastern-center of the study area in the Ocampo-Paraíso basin (Figure 1). The temperature measured in this sample was about 24.4°C and Cl- concentrations of 0.84. meq/L; 2.46 meq/L of Li+; and, 0.24 meq/L of Br- (Figure 7). The water type of this member is Na-K-HCO3.
Sub-basin III, is represented by sample 16. This water sample represents an intermediate flow and is located towards the north of the study area, towards the Jagüey de San Francisco, belonging to the basin of San Nicolás Tolentino (Figure 1). It has high temperatures of 20.5°C and have concentrations of Cl- of 0.819 meq/L; 0.312 meq/L of Li+; and high concentrations of Br- (1.67 meq/L) (Figure 7). This type of water is Na-K-HCO3.
In Table 2, C1 contributes 50 %, C2 contributes 31%, and C3 contributes 19% of water to the system (Table 2).
Table 2 Mixing fractions considering three extreme members (samples 30, 1 and 16) for the groundwater of the Zona Media of San Luis Potosí.
| Sample | C1 | C2 | C3 | CT |
| 1 | 0.00 | 1.00 | 0.00 | 1.00 |
| 2 | 0.01 | 0.20 | 0.79 | 1.00 |
| 3 | 0.63 | 0.24 | 0.13 | 1.00 |
| 4 | 0.74 | 0.25 | 0.01 | 1.00 |
| 5 | 0.64 | 0.17 | 0.19 | 1.00 |
| 6 | 0.35 | 0.31 | 0.33 | 1.00 |
| 7 | 0.27 | 0.64 | 0.09 | 1.00 |
| 8 | 0.44 | 0.54 | 0.02 | 1.00 |
| 9 | 0.35 | 0.55 | 0.11 | 1.00 |
| 10 | 0.44 | 0.52 | 0.04 | 1.00 |
| 11 | 0.15 | 0.71 | 0.14 | 1.00 |
| 12 | 0.87 | 0.03 | 0.09 | 1.00 |
| 13 | * | * | * | * |
| 14 | 0.57 | 0.35 | 0.08 | 1.00 |
| 15 | 0.54 | 0.38 | 0.07 | 1.00 |
| 16 | 0.00 | 0.00 | 1.00 | 1.00 |
| 17 | 0.48 | 0.39 | 0.13 | 1.00 |
| 18 | 0.58 | 0.44 | 0.00 | 1.00 |
| 19 | 0.44 | 0.42 | 0.14 | 1.00 |
| 20 | 0.72 | 0.28 | 0.00 | 1.00 |
| 21 | 0.72 | 0.01 | 0.27 | 1.00 |
| 22 | 0.75 | 0.20 | 0.05 | 1.00 |
| 23 | 0.75 | 0.21 | 0.03 | 1.00 |
| 24 | 0.63 | 0.29 | 0.08 | 1.00 |
| 25 | 0.81 | 0.15 | 0.04 | 1.00 |
| 26 | 0.40 | 0.26 | 0.35 | 1.00 |
| 27 | 0.00 | 0.09 | 0.91 | 1.00 |
| 28 | 0.74 | 0.26 | 0.00 | 1.00 |
| 29 | 0.77 | 0.23 | 0.00 | 1.00 |
| 30 | 1.00 | 0.00 | 0.00 | 1.00 |
| 31 | 0.94 | 0.02 | 0.03 | 1.00 |
| 32 | 0.73 | 0.27 | 0.00 | 1.00 |
| 33 | 0.86 | 0.13 | 0.01 | 1.00 |
| 34 | 0.71 | 0.17 | 0.12 | 1.00 |
Conclusions
The aquifer receives its recharge water that drains through flat platform rocks (El Abra Formation), gypsum, anhydrite (Guaxcama Formation) and volcanic rocks that delimit the aquifer.
Three different types calcium bicarbonate (Ca-HCO3), sodium bicarbonate (Na-HCO3), calcium sulfate (Ca-SO4) waters were obtained.
A local and an intermediate regional flow were identified, where each system has a representative sample used as an extra limb.
A ternary mixture and water-rock interaction processes were identified in the study area.
The system is fed by three essential regions. C1, C2 and C3 contributes 50%, 31% and 19%, respectively of water to the system.











 text new page (beta)
text new page (beta)